Speak directly to the analyst to clarify any post sales queries you may have.
A concise strategic framing of the evolving pharmacy benefit management sector highlighting clinical, financial, and regulatory priorities for executives
The pharmacy benefit management environment has entered a phase of intensified scrutiny, strategic repositioning, and technological acceleration that requires leaders to re-evaluate long-standing assumptions about contracting, benefit design, and clinical oversight. The industry is being reshaped by converging forces: payers and employers demanding greater transparency and outcomes alignment, regulators imposing new disclosure and pricing requirements, and providers experimenting with integrated care models that shift where and how medications are accessed. Against this backdrop, pharmacy benefit managers remain central intermediaries, but their operating models and value propositions are under renewed examination.Consequently, executives must prioritize a clearer articulation of how their organizations deliver measurable cost control, quality improvement, and patient experience gains. This introduction sets the stage for a structured analysis that explores not only the operational and commercial levers available to pharmacy benefit managers but also the macro drivers-policy shifts, global supply chain pressures, and advances in analytics-that influence strategic choices. By framing the debate in terms of clinical outcomes, financial integrity, and member-centered services, the following sections provide the actionable context leaders need to make deliberate, evidence-informed decisions.
How regulatory transparency, value-based contracting, and digital clinical capabilities are collectively reshaping competitive advantage in pharmacy benefit management
The landscape for pharmacy benefit management is undergoing transformative shifts driven by policy reform, value-based care adoption, and heightened expectations for transparency and member engagement. New regulations and public scrutiny have increased the demand for visible pricing mechanisms and predictable contracting structures, which in turn are prompting PBMs, payers, and employers to pilot alternative models that emphasize pass-through pricing, administrative fees, and performance-linked arrangements. As stakeholders test these structures, the competitive field is recalibrating: established incumbents refine their offerings while new entrants and vertically integrated health systems pursue differentiated value propositions.Technological advances are amplifying these strategic shifts. Sophisticated analytics, real-time claims adjudication engines, and AI-enabled utilization tools are enabling more precise clinical interventions and tighter cost control. In parallel, specialty drugs and complex care regimens continue to pressure benefit design and distribution strategies, necessitating innovative clinical support services and closer coordination with specialty pharmacies. Taken together, these trends are not incremental; they represent a systemic evolution where contractual transparency, clinical performance, and digital capabilities collectively determine competitive advantage and operational resilience.
Trade-driven pressures in 2025 forcing procurement agility, supplier diversification, and contract innovations that protect benefit integrity and patient access
Tariff policy changes and international trade adjustments in 2025 have introduced an additional layer of complexity into pharmaceutical supply chains that indirectly affect pharmacy benefit management programs. Increased tariffs on certain pharmaceutical components and logistical goods have elevated attention to sourcing strategies, inventory management, and cross-border procurement practices. These pressures have led plan sponsors and PBMs to reassess supplier diversification, to intensify inventory optimization, and to explore alternative fulfillment models that mitigate exposure to tariff-driven cost variability.As a result, contractual provisions and formulary strategies have been adapted to reflect supply chain uncertainty and potential price volatility. Pharmacy benefit leaders are increasingly incorporating contingency clauses, alternative supplier arrangements, and dynamic rebate negotiation mechanisms to preserve predictability for plan sponsors and patients. Moreover, the tariff environment has accelerated interest in domestic manufacturing incentives and in partnerships that secure steady supply of high-value specialty therapies. In short, trade-related changes in 2025 have reinforced the importance of agile procurement, robust supplier risk management, and the alignment of clinical pathways with resilient distribution networks.
A multidimensional segmentation framework tying customer type, drug type, distribution channel, service offering, contract and revenue models, ownership, size, and technology together
Understanding how market value is created requires a granular view across multiple segmentation dimensions that influence contracting, clinical design, and distribution strategies. Based on customer type, stakeholders range from employers segmented into large employers, mid market employers, and small employers, to government programs that include Medicaid programs and Medicare Part D plans, and to individuals accessing coverage through individual plans and exchanges; each cohort demands tailored administrative services, pricing transparency, and outcomes reporting. Based on drug type, commercial and clinical strategies must differentiate among brand prescription drugs, generic drugs, and specialty drugs, with specialty further broken down into autoimmune, oncology, and rare disease therapies that require elevated clinical management and specialty dispensing capabilities.Based on distribution channel, program efficacy rests on long term care pharmacy, mail order and home delivery models, retail pharmacy network relationships, and specialty pharmacy operations that include onsite specialty dispensing and broader specialty pharmacy networks; channel choices significantly affect adherence, continuity of care, and total cost of therapy. Based on service offering, the market differentiates administrative services such as claims processing and adjudication from clinical programs like formulary management and utilization management, alongside clinical support services exemplified by medication therapy management and specialty services including specialty drug dispensing. Contract model distinctions-hybrid discount, pass-through administrative fee, and spread pricing-shape revenue transparency expectations and define sponsor risk exposure. Revenue models vary between administrative fees, performance-based fees, rebates and manufacturer discounts, and spread, each carrying implications for alignment with sponsor incentives. Ownership models such as health plan owned entities, independent PBMs, pharmacy chain owned operators, and vertically integrated healthcare systems influence strategic priorities around integration and service breadth. Customer size categories from large employers with 1,000-plus employees to mid-market organizations and small employers under 100 dictate scale economics and service customization. Finally, technology and analytics capabilities encompass advanced analytics, claims adjudication systems, and technology platforms, where advanced analytics includes clinical analytics and AI-driven predictive utilization tools and technology platforms comprise benefit administration and specialty pharmacy platforms that enable operational scale and clinical decision support. Integrating these segmentation lenses provides a multidimensional framework for executives to evaluate where to invest, which partnerships to cultivate, and how to redesign contracts and clinical workflows to drive measurable improvements across populations and product types.
How distinct regional regulatory, payer, and supply chain conditions across the Americas, Europe Middle East and Africa, and Asia-Pacific shape differentiated PBM strategies
Regional dynamics exert pronounced influence on regulatory frameworks, payer expectations, and the availability of clinical and distribution infrastructure that supports pharmacy benefit management. In the Americas, regulatory scrutiny and employer-led innovation have intensified, with stakeholders prioritizing transparency, specialty drug management, and employer-driven plan design experimentation that seeks to reconcile cost control with employee access and outcomes. In Europe, Middle East & Africa, varied regulatory regimes and differing reimbursement norms necessitate tailored strategies that account for national formularies, public payer structures, and heterogeneous distribution models, while private payers and integrated care systems in some markets are piloting value-based pharmaceutical contracts.Across Asia-Pacific, rapid adoption of digital health tools, evolving payer-provider collaborations, and growing local biopharmaceutical manufacturing capacity create opportunities for localized specialty distribution models and partnerships focused on adherence and patient support. These regional distinctions influence how PBMs and plan sponsors approach supplier contracting, clinical program design, and technology investments, and they underline the need for market-entry strategies that are sensitive to regulatory nuance, payment flows, and the maturity of retail and specialty pharmacy channels. Thus, regional insight is essential for tailoring service offerings and for anticipating regulatory and supply-side shifts that affect program performance.
Competitive dynamics and strategic moves among legacy administrators, vertically integrated systems, specialty operators, and technology-first entrants defining industry leadership
The competitive landscape includes legacy administrators, vertically integrated health systems, specialty pharmacy operators, and nimble technology-first entrants that are redefining expectations for clinical engagement and commercial transparency. Key companies are investing in end-to-end capabilities that span benefit administration, specialty dispensing, clinical analytics, and member-facing services. Several market leaders emphasize scale in claims adjudication and rebate negotiation, while others pursue vertical integration to control distribution and specialty logistics, and yet another cohort focuses on advanced analytics and AI to differentiate clinical outcomes and utilization management.Across these varied strategies, common themes emerge: firms that successfully demonstrate improvements in clinical adherence, transparent contracting, and reductions in avoidable spend are gaining traction with large employers and complex payers. Partnerships and strategic alliances are increasingly used to complement internal capabilities, especially to address specialty therapy management and last-mile fulfillment. Investors and acquirers remain attentive to businesses that can show durable clinical value, predictable operational margins, and technology architectures that support rapid integration and continuous improvement.
Practical prioritized measures for payers and administrators to enhance transparency, integrate analytics, fortify supply chains, and drive measurable clinical improvements
Industry leaders should prioritize a sequence of actions that enhance transparency, clinical impact, and operational resilience. First, aligning contract models with sponsor expectations is essential; organizations should evaluate opportunities to transition toward pass-through or hybrid arrangements where appropriate, while ensuring compensation aligns with demonstrable clinical value and improved patient outcomes. Second, investments in advanced analytics and AI should be targeted to clinical use cases such as adherence prediction, early intervention for specialty therapy risks, and optimized formulary placement; these capabilities must be integrated into workflows and decision support rather than treated as standalone projects.Third, strengthening supply chain and procurement strategies will mitigate tariff and trade-related risks; leaders should diversify sourcing, implement supplier performance provisions, and design fulfillment options that balance cost, access, and continuity. Fourth, enhancing specialty pharmacy partnerships and expanding clinical support services-particularly medication therapy management and onsite specialty dispensing-will improve outcomes for complex patients. Finally, governance and measurement frameworks must be elevated so that contract performance, clinical outcomes, and member experience are tracked with rigorous KPIs and tied to compensation where possible. Taken together, these recommendations provide a practical roadmap to shift from transactional administration to outcome-oriented pharmacy benefit management.
A rigorous mixed methods research approach combining industry interviews, regulatory review, and scenario analysis to validate strategic implications and recommendations
This research synthesis was developed through a rigorous mixed-methods approach integrating qualitative and quantitative inputs to yield a comprehensive view of operational realities and strategic trends. Primary research included structured interviews with plan executives, pharmacy directors, and provider leaders to understand contracting preferences, clinical program efficacy, and distribution pain points. Secondary analysis drew on publicly available regulatory documents, industry white papers, and vendor technical specifications to triangulate findings and to ensure interpretations align with the observable regulatory and technological landscape.Analytical methods included thematic coding of qualitative interviews to identify recurring governance and service model priorities, and comparative analysis of contract structures and service offerings to highlight differentiators. Scenario-based thinking was applied to evaluate the downstream implications of tariff shifts and regulatory reforms on procurement and formulary strategies. Throughout, emphasis was placed on cross-validating insights across stakeholder groups to reduce individual bias and to present recommendations that are actionable for both commercial sponsors and clinical operations teams.
A concise synthesis of how transparency, advanced analytics, contract realignment, and supply chain resilience collectively determine future competitiveness in the PBM sector
In summary, the pharmacy benefit management sector stands at an inflection point where regulatory scrutiny, clinical complexity driven by specialty therapies, evolving contract models, and supply chain pressures converge to demand strategic recalibration. Success will hinge on the ability of organizations to demonstrate transparent commercial practices, to integrate advanced analytics into clinical workflows, and to design distribution and specialty support that safeguards access and optimizes outcomes. Stakeholders who align contract incentives with measurable clinical and financial goals, while investing in digital platforms that enable real-time adjudication and predictive utilization management, will be best positioned to capture durable advantage.As market participants navigate these shifts, operational discipline in procurement, heightened clinical engagement for specialty populations, and governance that ties performance to compensation will separate leading entities from those that remain transactional. Executives should treat this period as an opportunity to redesign how pharmacy benefits are delivered and governed, moving decisively from reactive management to proactive, outcome-focused stewardship of medication use and patient care.
Market Segmentation & Coverage
This research report forecasts the revenues and analyzes trends in each of the following sub-segmentations:- Service Offering
- Analytics & Reporting
- Client Dashboards
- Fraud, Waste, & Abuse Detection
- Population Insights
- Quality & Star Ratings Support
- Claims Administration
- Billing & Reconciliation
- Coordination of Benefits
- Eligibility & Enrollment
- Real-Time Adjudication
- Clinical Management
- Adherence Programs
- Case Management
- Disease Management
- Medication Therapy Management
- Formulary Management
- P&T Committee Support
- Tier Strategy
- Utilization Controls
- Member & Provider Support
- Appeals & Grievances
- Member Services
- Provider Helpdesk
- Network Management
- Mail Service Operations
- Performance-Based Contracting
- Retail Contracting
- Specialty Network
- Rebate Management
- Contract Administration
- Outcomes-Based Agreements
- Rebate Negotiation
- Reconciliation & Audit
- Analytics & Reporting
- Ownership Model
- Health Plan Owned
- Independent PBMs
- Pharmacy Chain Owned
- Vertically Integrated Healthcare Systems
- Therapeutic Class
- Antidepressants, anxiety treatments
- Chronic Disease Drugs
- Diabetes, cardiovascular, respiratory diseases
- High-cost biologics, gene therapy treatments
- Mental Health & CNS Drugs
- Oncology & Specialty Drugs
- Opioids, non-opioid therapies
- Pain Management
- Preventive & Vaccination Drugs
- Pharmacy Channel
- Digital Pharmacy
- Health System Outpatient
- Home Delivery
- Long-Term Care
- Mail Order
- Retail
- Chain Pharmacies
- Independent Pharmacies
- Mass Merchandiser & Supermarket
- Specialty Pharmacy
- Client Type
- Employers
- Large Employers
- Small & Medium Employers
- Government Programs
- Medicaid
- Fee-For-Service
- Managed Medicaid
- Medicare
- Medicare Advantage Prescription Drug
- Part D Prescription Drug Plans
- Military & Federal
- Federal Employee Health Benefits
- TRICARE
- Medicaid
- Health Plans
- Provider-Sponsored Plans
- Third-Party Administrators
- Unions & Trusts
- Employers
- Americas
- North America
- United States
- Canada
- Mexico
- Latin America
- Brazil
- Argentina
- Chile
- Colombia
- Peru
- North America
- Europe, Middle East & Africa
- Europe
- United Kingdom
- Germany
- France
- Russia
- Italy
- Spain
- Netherlands
- Sweden
- Poland
- Switzerland
- Middle East
- United Arab Emirates
- Saudi Arabia
- Qatar
- Turkey
- Israel
- Africa
- South Africa
- Nigeria
- Egypt
- Kenya
- Europe
- Asia-Pacific
- China
- India
- Japan
- Australia
- South Korea
- Indonesia
- Thailand
- Malaysia
- Singapore
- Taiwan
- CVS Health Corporation
- Cigna Corporation
- Abarca Health LLC
- Alluma LLC
- AmWINS Group Benefits, LLC
- Benecard Services LLC
- Cadence Rx, Inc.
- CaptureRx
- Change Healthcare Technologies, LLC
- ClearScript, Inc.
- Elevance Health, Inc.
- Humana Inc.
- IllarumRx, LLC
- Kroger Prescription Plans, Inc.
- MaxorPlus, Ltd.
- MedImpact Healthcare Systems, Inc.
- Navitus Health Solutions, LLC
- OptumRx, Inc.
- Phoenix Benefits Management, LLC
- Prime Therapeutics LLC
- ProAct, Inc.
- ProCare Pharmacy Benefit Manager, Inc.
- Serve You Rx, LLC
- SS&C Technologies, Inc.
- UnitedHealth Group Incorporated
- US Rx Care, LLC
Table of Contents
3. Executive Summary
4. Market Overview
7. Cumulative Impact of Artificial Intelligence 2025
Companies Mentioned
The companies profiled in this Pharmacy Benefit Management market report include:- CVS Health Corporation
- Cigna Corporation
- Abarca Health LLC
- Alluma LLC
- AmWINS Group Benefits, LLC
- Benecard Services LLC
- Cadence Rx, Inc.
- CaptureRx
- Change Healthcare Technologies, LLC
- ClearScript, Inc.
- Elevance Health, Inc.
- Humana Inc.
- IllarumRx, LLC
- Kroger Prescription Plans, Inc.
- MaxorPlus, Ltd.
- MedImpact Healthcare Systems, Inc.
- Navitus Health Solutions, LLC
- OptumRx, Inc.
- Phoenix Benefits Management, LLC
- Prime Therapeutics LLC
- ProAct, Inc.
- ProCare Pharmacy Benefit Manager, Inc.
- Serve You Rx, LLC
- SS&C Technologies, Inc.
- UnitedHealth Group Incorporated
- US Rx Care, LLC
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 192 |
| Published | November 2025 |
| Forecast Period | 2025 - 2032 |
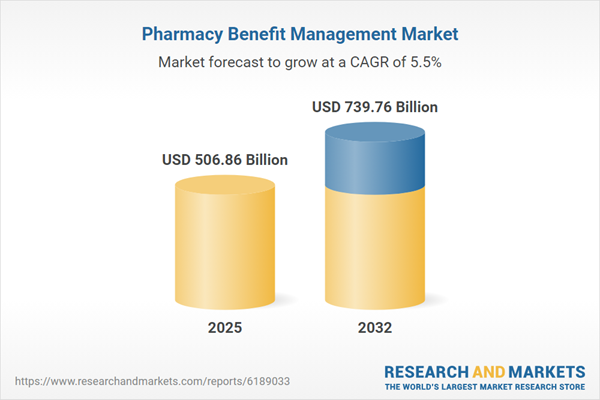
| Estimated Market Value ( USD | $ 506.86 Billion |
| Forecasted Market Value ( USD | $ 739.76 Billion |
| Compound Annual Growth Rate | 5.5% |
| Regions Covered | Global |
| No. of Companies Mentioned | 27 |