Approximately one-third of people 65 and older have chronic kidney disease (CKD), compared to about 12% of people 45 to 64 and only 6% of adults 18 to 44, according to data released by the CDC in 2024. The prevalence is slightly higher in women (14%), than in men (12%). Significant racial and ethnic disparities also exist: the highest prevalence is found among non-Hispanic Black adults (20%), followed by non-Hispanic Asian and Hispanic adults (14% each), and non-Hispanic White adults (12%). The data highlights the critical need for enhanced screening programs and early intervention strategies.
The U.S. renal biomarkers market is growing due to the rising prevalence of CKD and increasing number of the laboratory automation, clear FDA pathways, and insurer willingness to reimburse novel tests that meet evidentiary thresholds. Test ordering frequency has increased, and protocols have been updated as a result of Medicare's Merit-based Incentive Payment System, which ties nephrology practice scores to annual albuminuria rates. The market is expanding as a result of the active development and commercialization of renal biomarker assays by major players such as Abbott Laboratories and bioMérieux SA. Recently, in August 2025, BioPorto and Roche Diagnostics collaboration: The ProNephro AKI test (based on NGAL biomarker) has become commercially available to U.S. labs via Roche’s cobas c 501 analyzers. NGAL is a protein that indicates kidney cell damage and can detect injury earlier than standard tests such as serum creatinine. The test is cleared for pediatric use (ages 3 months to 21 years), helps identify moderate-to-severe acute kidney injury (AKI) within 48-72 hours, enabling earlier interventions.
Moreover, as per CDC data published in May 2024, CDC estimating that more than 1 in 7 adults-around 35.5 million people, or 14% of the adult population are affected in U.S. Alarmingly, CKD often goes undiagnosed: as many as 9 in 10 adults with CKD are unaware of their condition, and even among those with severe CKD, approximately one in three remain undiagnosed.
U.S. Renal Biomarkers Market Report Segmentation
This report forecasts revenue growth and provides an analysis of the latest trends in each of the sub-segments from 2021 to 2033. For this study, the analyst has segmented the U.S. Renal Biomarkers market based on biomarker, diagnostic technique/platform, and end-use:Biomarkers Outlook (Revenue, USD Million, 2021-2033)
- Functional Biomarkers
- Upregulated Proteins
- Other Novel Biomarkers
Diagnostic technique /Platform Outlook (Revenue, USD Million, 2021-2033)
- Enzyme-Linked Immunosorbent Assay (ELISA)
- Immunoassays
- Clinical Chemistry Assays
- Point-of-Care Testing (POCT) Devices
- Molecular Diagnostics
- Other Emerging Platforms
End-use Outlook (Revenue, USD Million, 2021-2033)
- Hospitals & Clinics
- Diagnostic Laboratories
- Academic & Research Institutes
- Pharmaceutical & Biotechnology Companies
- Others
Why You Should Buy This Report
- Comprehensive Market Analysis: Gain detailed insights into the market across major regions and segments.
- Competitive Landscape: Explore the market presence of key players.
- Future Trends: Discover the pivotal trends and drivers shaping the future of the market.
- Actionable Recommendations: Utilize insights to uncover new revenue streams and guide strategic business decisions.
Report Deliverables
- Market intelligence to enable effective decision-making
- Market estimates and forecasts from 2018 to 2030
- Growth opportunities and trend analyses
- Segment and regional revenue forecasts for market assessment
- Competition strategy and market share analysis
- Product innovation listings for you to stay ahead of the curve
This product will be delivered within 2 business days.
Table of Contents
Companies Mentioned
The companies profiled in this U.S. Renal Biomarkers market report include:- Thermo Fisher Scientific Inc.
- Siemens Healthineers AG
- BioPorto Diagnostics A/S
- SEKISUI Medical Co., Ltd.
- BioMerieux SA
- SphingoTec GmbH
- Randox Laboratories Ltd
- Beckman Coulter Inc.
- QIAGEN N.V.
- Becton, Dickinson & Co.
- DiaSorin S.p.A.
- Bio-Rad Laboratories Inc.
- EKF Diagnostics Holdings plc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 150 |
| Published | October 2025 |
| Forecast Period | 2024 - 2033 |
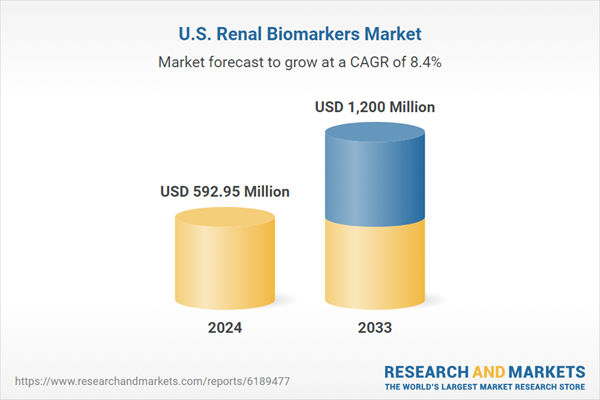
| Estimated Market Value ( USD | $ 592.95 Million |
| Forecasted Market Value ( USD | $ 1200 Million |
| Compound Annual Growth Rate | 8.4% |
| Regions Covered | United States |
| No. of Companies Mentioned | 14 |