Cardiac Implant Devices Market Analysis
The cardiac implant devices market is a vital segment of the medical device industry, driven by the rising prevalence of cardiovascular diseases and an aging global population. These devices, including pacemakers, implantable cardioverter defibrillators (ICDs), and coronary stents, address critical needs in managing heart conditions. Technological advancements and supportive healthcare policies fuel growth, though high costs pose challenges.This research examines current trends in demand, supply, and sales, alongside recent developments shaping the cardiac implant devices market. It provides a comprehensive analysis of key drivers, restraints, and opportunities, detailing industry trends, policies, and regulations across geographical regions to equip stakeholders with insights into the regulatory framework and market dynamics.
Competitive intelligence identifies major industry players and their revenue contributions, derived from extensive secondary research, including industry association studies, analyst reports, investor presentations, press releases, and journals. Market size for the overall sector and key segments was determined using bottom-up and top-down methodologies, validated with primary inputs from stakeholders in the global cardiac implant devices value chain. Comprehensive market engineering integrated data from diverse sources and proprietary datasets, employing data triangulation for accurate market breakdown and forecasting. Insights are presented through analytical narratives, charts, and graphics for efficient comprehension. Key players profiled include leading manufacturers of pacemakers, ICDs, and coronary stents.
Key Highlights
- Cardiovascular Disease Prevalence: Rising cases, affecting 600 million globally in 2024, drive demand for cardiac implants.
- Aging Population: A geriatric population of 1.5 billion by 2050 fuels heart disease-related device needs.
- Technological Advancements: Innovations in device design enhance efficacy, boosting market growth.
- Cost Barriers: High device costs, averaging USD 20,000-50,000, limit adoption in cost-sensitive markets.
Growth Drivers
The increasing prevalence of cardiovascular diseases, with a 5% rise in cases in 2024, significantly boosts demand for devices like coronary stents and ICDs. An aging population, particularly in North America and Europe, drives heart failure and arrhythmia cases, necessitating implants. Technological advancements, such as leadless pacemakers introduced in 2024, improve patient outcomes and adoption. Supportive government initiatives, like India’s USD 1 billion healthcare access program in 2024, enhance affordability in APAC.Restraints
High costs of cardiac implant devices, coupled with implantation procedures, restrict access in emerging markets. Stringent regulatory approvals, such as FDA and EU MDR requirements tightened in 2024, increase development timelines. Limited reimbursement policies in developing regions hinder market penetration.Segmentation Analysis
By Product Type: Coronary stents lead with a 30% share in 2024, driven by coronary heart disease prevalence. Implantable heart pumps grow rapidly due to rising heart failure cases.By Application: Arrhythmia management dominates, followed by heart failure and coronary artery disease treatments.
By End-User: Hospitals hold the largest share, with outpatient clinics expanding for minimally invasive procedures.
Regional Analysis
North America commands a 40% market share in 2024, driven by high disposable incomes and advanced healthcare infrastructure in the U.S. Asia-Pacific grows at a 9.2% CAGR, fueled by rising cardiovascular cases and healthcare investments in China and India. Europe sustains growth through robust healthcare systems and R&D investments.This report equips industry experts with critical insights into market trends, regulatory landscapes, and competitive dynamics. It highlights opportunities in advanced device technologies and emerging markets while addressing cost and regulatory challenges. The rigorous methodology, blending primary and secondary data, ensures reliable projections, enabling stakeholders to navigate complexities and prioritize investments in this essential medical device sector.
Key Benefits of this Report:
- Insightful Analysis: Gain detailed market insights covering major as well as emerging geographical regions, focusing on customer segments, government policies and socio-economic factors, consumer preferences, industry verticals, and other sub-segments.
- Competitive Landscape: Understand the strategic maneuvers employed by key players globally to understand possible market penetration with the correct strategy.
- Market Drivers & Future Trends: Explore the dynamic factors and pivotal market trends and how they will shape future market developments.
- Actionable Recommendations: Utilize the insights to exercise strategic decisions to uncover new business streams and revenues in a dynamic environment.
- Caters to a Wide Audience: Beneficial and cost-effective for startups, research institutions, consultants, SMEs, and large enterprises.
What do businesses use our reports for?
Industry and Market Insights, Opportunity Assessment, Product Demand Forecasting, Market Entry Strategy, Geographical Expansion, Capital Investment Decisions, Regulatory Framework & Implications, New Product Development, and Competitive Intelligence.Report Coverage:
- Historical data from 2022 to 2024 & forecast data from 2025 to 2030
- Growth Opportunities, Challenges, Supply Chain Outlook, Regulatory Framework, and Trend Analysis
- Competitive Positioning, Strategies, and Market Share Analysis
- Revenue Growth and Forecast Assessment of segments and regions including countries
- Company Profiling (Strategies, Products, Financial Information, and Key Developments among others)
Segmentation
- By Product Type
- Pacemakers
- Cardiac Resynchronization Therapy (CRT) devices
- Implantable Cardioverter Defibrillator (ICD)
- Transcatheter Heart Valves (TAVR/TAVI) & Surgical Prosthetic Valves
- Ventricular Assist Devices (VADs/LVADs)
- Cardiac Monitoring Implants
- Left Atrial Appendage (LAA) Closure Device
- Coronary Stents
- Prosthetic Heart Valves
- Insertable Cardiac Monitor (ICM)
- Implantable Heart Pump
- Leads, Generators, and Delivery Systems
- Others
- By Distribution
- Tertiary Hospitals
- Community Hospitals
- Ambulatory Surgery Centers
- Cardiac Clinics
- By Geography
- North America
- USA
- Canada
- Mexico
- South America
- Brazil
- Argentina
- Others
- Europe
- United Kingdom
- Germany
- France
- Italy
- Spain
- Others
- Middle East and Africa
- Saudi Arabia
- UAE
- Others
- Asia-Pacific
- China
- Japan
- India
- South Korea
- Taiwan
- Thailand
- Indonesia
- Others
- North America
Table of Contents
Companies Mentioned
- Medtronic plc
- Abbott Laboratories
- Boston Scientific Corporation
- Edwards Lifesciences Corporation
- Biotronik SE & Co. KG
- LivaNova PLC
- Lepu Medical Technology Co., Ltd.
- Asahi Kasei Corporation (Zoll Medical)
- Abiomed, Inc.
- SynCardia Systems, LLC
- MicroPort Scientific Corporation:
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 143 |
| Published | November 2025 |
| Forecast Period | 2025 - 2030 |
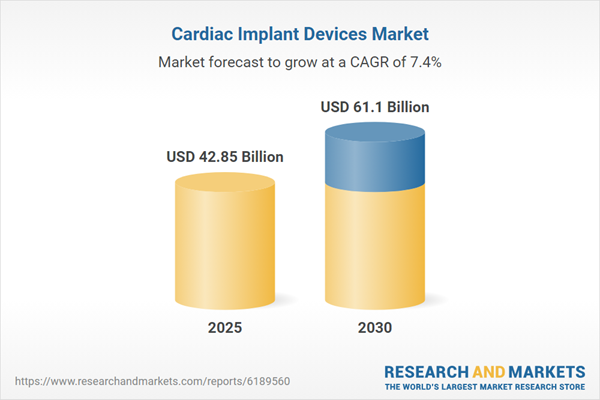
| Estimated Market Value ( USD | $ 42.85 Billion |
| Forecasted Market Value ( USD | $ 61.1 Billion |
| Compound Annual Growth Rate | 7.3% |
| Regions Covered | Global |
| No. of Companies Mentioned | 11 |