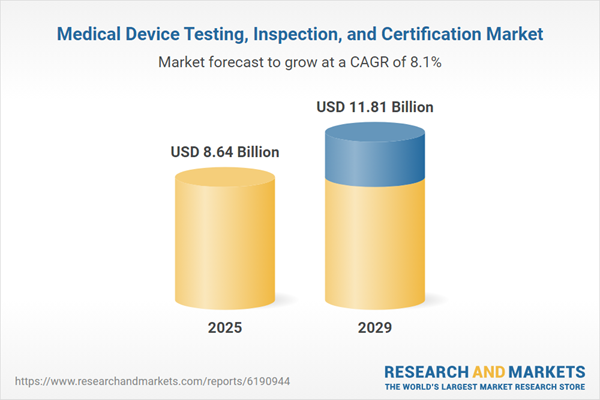
The medical device testing, inspection, and certification market size is expected to see strong growth in the next few years. It will grow to $11.81 billion in 2029 at a compound annual growth rate (CAGR) of 8.1%. Growth in the forecast period can be attributed to increasing regulatory compliance requirements for medical devices, growing demand for high-quality and safe medical products, rising adoption of advanced testing and inspection technologies, greater emphasis on risk management and patient safety, and increasing investment in medical device research and development. Key trends expected during this period include advancements in automated and robotics-based inspection systems, the use of artificial intelligence for predictive quality analysis, innovation in non-destructive testing techniques, integration of digital twins for device performance validation, and progress in the global harmonization of certification standards.
The increasing healthcare expenditures are expected to drive the growth of the medical device testing, inspection, and certification market in the coming years. Healthcare expenditures refer to the financial resources allocated by governments, private organizations, or individuals for healthcare services, treatments, and related activities. These expenditures are rising due to the growing prevalence of chronic diseases that require prolonged and costly medical care. Increased healthcare spending supports medical device testing, inspection, and certification by funding the development, quality assurance, and regulatory compliance of safe and effective medical technologies. For example, in May 2024, the Office for National Statistics, a UK-based government department, reported that total healthcare expenditure grew by 5.6% in nominal terms from 2022 to 2023, marking a significant acceleration compared with the 0.9% growth recorded in 2022. Therefore, the rise in healthcare expenditures is contributing to the expansion of the medical device testing, inspection, and certification market.
Major players in the medical device testing, inspection, and certification market are focusing on enhancing their service offerings through advanced third-party verification and validation solutions to strengthen trust and ensure compliance. Third-party verification and validation involve an independent organization assessing and confirming that a product, process, or system meets established standards or requirements. For instance, in October 2023, UL Solutions, a US-based provider of testing, inspection, and certification services, launched medical device testing services at its laboratory in Rochester Hills, Michigan. These new services aim to support the state’s growing medical device industry by improving product safety, security, usability, and interoperability. The Rochester Hills facility provides comprehensive third-party verification and validation, auditing, cybersecurity evaluation, usability testing, and compliance training for manufacturers. It also offers flexible testing capabilities, including accelerated lifespan and environmental testing, tailored to specific manufacturer needs. Additionally, the facility maintains a controlled environment with low volatile organic compound (VOC) levels to ensure testing precision and reliability.
In November 2024, Applus+, a Spain-based provider of testing, inspection, and certification services, acquired Keystone Compliance for an undisclosed amount. This acquisition aims to enhance Applus+’s presence in the North American market by expanding its local testing capabilities and providing comprehensive product testing and certification services across multiple industries. Keystone Compliance is a US-based company specializing in electromagnetic compatibility (EMC/EMI), environmental, and packaging testing services for clients in the electronics, medical device, aerospace, defense, and energy sectors.
Major players in the medical device testing, inspection, and certification market are Société Générale de Surveillance S.A., Eurofins Scientific S.E., Bureau Veritas S.A., Intertek Group plc, Charles River Laboratories International Inc., TÜV SÜD Aktiengesellschaft, UL Solutions Inc., Element Materials Technology Limited, QIMA Limited, Cotecna Inspection SA, North American Science Associates LLC, TÜV Rheinland AG, Freyr Solutions Pvt. Ltd., Nelson Laboratories LLC, MED Institute Inc., Auriga Research Limited, DDL Inc., F2 Labs LLC, DeviceLab LLC, and EdgeOne Medical Inc.
North America was the largest region in the medical device testing, inspection, and certification market in 2024. Asia-Pacific is expected to be the fastest-growing region in the forecast period. The regions covered in medical device testing, inspection, and certification report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East and Africa.
Note that the outlook for this market is being affected by rapid changes in trade relations and tariffs globally. The report will be updated prior to delivery to reflect the latest status, including revised forecasts and quantified impact analysis. The report’s Recommendations and Conclusions sections will be updated to give strategies for entities dealing with the fast-moving international environment.
The sudden escalation of U.S. tariffs and the consequent trade frictions in spring 2025 are severely impacting the healthcare sector, particularly in the supply of critical medical devices, diagnostic equipment, and pharmaceuticals. Hospitals and healthcare providers are facing higher costs for imported surgical instruments, imaging equipment, and consumables such as syringes and catheters, many of which have limited domestic alternatives. These increased costs are straining healthcare budgets, leading some providers to delay equipment upgrades or pass on expenses to patients. Additionally, tariffs on raw materials and components are disrupting the production of essential drugs and devices, causing supply chain bottlenecks. In response, the industry is diversifying sourcing strategies, boosting local manufacturing where possible, and advocating for tariff exemptions on life-saving medical products.
The medical device testing, inspection, and certification market research report is one of a series of new reports that provides medical device testing, inspection, and certification market statistics, including the medical device testing, inspection, and certification industry global market size, regional shares, competitors with the medical device testing, inspection, and certification market share, detailed medical device testing, inspection, and certification market segments, market trends, and opportunities, and any further data you may need to thrive in the medical device testing, inspection, and certification industry. This medical device testing, inspection, and certification market research report delivers a complete perspective of everything you need, with an in-depth analysis of the current and future scenarios of the industry.
Medical device testing, inspection, and certification is a comprehensive process designed to ensure that medical devices comply with safety, quality, performance, and regulatory standards. It includes functional testing, biocompatibility assessment, reliability evaluation, and certification in line with international regulations, ensuring that devices are safe, effective, and compliant before being introduced to healthcare providers or patients.
The primary device types involved in medical device testing, inspection, and certification include active medical devices, non-active medical devices, in-vitro diagnostic devices, and combination products. Active medical devices are those that require an electrical energy source or another power supply to function. These devices undergo various testing processes such as safety, performance, biocompatibility, and efficacy testing, as well as inspection procedures including component inspection, final product inspection, quality system inspection, and compliance inspection. Certification for these devices may include International Organization for Standardization (ISO) certification, Conformité Européenne (CE) marking, Food and Drug Administration (FDA) approval, and other regulatory validations. The main end users of these processes include medical device manufacturers, regulatory authorities, healthcare institutions, and independent testing laboratories.
The countries covered in the medical device testing, inspection, and certification market report are Australia, Brazil, China, France, Germany, India, Indonesia, Japan, Russia, South Korea, UK, USA, Canada, Italy, Spain.
The medical device testing, inspection, and certification market includes revenues earned by entities by providing services such as risk assessment services, sterilization validation, software validation, packaging integrity evaluation, and environmental simulation testing. The market value includes the value of related goods sold by the service provider or included within the service offering. Only goods and services traded between entities or sold to end consumers are included.
The market value is defined as the revenues that enterprises gain from the sale of goods and/or services within the specified market and geography through sales, grants, or donations in terms of the currency (in USD unless otherwise specified).
The revenues for a specified geography are consumption values that are revenues generated by organizations in the specified geography within the market, irrespective of where they are produced. It does not include revenues from resales along the supply chain, either further along the supply chain or as part of other products.
This product will be delivered within 3-5 business days.
Table of Contents
Executive Summary
Medical Device Testing, Inspection, and Certification Global Market Report 2025 provides strategists, marketers and senior management with the critical information they need to assess the market.This report focuses on medical device testing, inspection, and certification market which is experiencing strong growth. The report gives a guide to the trends which will be shaping the market over the next ten years and beyond.
Reasons to Purchase:
- Gain a truly global perspective with the most comprehensive report available on this market covering 15 geographies.
- Assess the impact of key macro factors such as geopolitical conflicts, trade policies and tariffs, post-pandemic supply chain realignment, inflation and interest rate fluctuations, and evolving regulatory landscapes.
- Create regional and country strategies on the basis of local data and analysis.
- Identify growth segments for investment.
- Outperform competitors using forecast data and the drivers and trends shaping the market.
- Understand customers based on the latest market shares.
- Benchmark performance against key competitors.
- Suitable for supporting your internal and external presentations with reliable high quality data and analysis
- Report will be updated with the latest data and delivered to you along with an Excel data sheet for easy data extraction and analysis.
- All data from the report will also be delivered in an excel dashboard format.
Description
Where is the largest and fastest growing market for medical device testing, inspection, and certification? How does the market relate to the overall economy, demography and other similar markets? What forces will shape the market going forward, including technological disruption, regulatory shifts, and changing consumer preferences? The medical device testing, inspection, and certification market global report answers all these questions and many more.The report covers market characteristics, size and growth, segmentation, regional and country breakdowns, competitive landscape, market shares, trends and strategies for this market. It traces the market’s historic and forecast market growth by geography.
- The market characteristics section of the report defines and explains the market.
- The market size section gives the market size ($b) covering both the historic growth of the market, and forecasting its development.
- The forecasts are made after considering the major factors currently impacting the market. These include: the technological advancements such as AI and automation, Russia-Ukraine war, trade tariffs (government-imposed import/export duties), elevated inflation and interest rates.
- Market segmentations break down the market into sub markets.
- The regional and country breakdowns section gives an analysis of the market in each geography and the size of the market by geography and compares their historic and forecast growth.
- The competitive landscape chapter gives a description of the competitive nature of the market, market shares, and a description of the leading companies. Key financial deals which have shaped the market in recent years are identified.
- The trends and strategies section analyses the shape of the market as it emerges from the crisis and suggests how companies can grow as the market recovers.
Report Scope
Markets Covered:
1) By Device Type: Active Medical Devices; Non-active Medical Devices; In-vitro Diagnostic Devices; Combination Products2) By Testing Type: Safety Testing; Performance Testing; Biocompatibility Testing; Efficacy Testing
3) By Inspection Type: Component Inspection; Final Product Inspection; Quality System Inspection; Compliance Inspection
4) By Certification Type: International Organization For Standardization (ISO) Certification; Conformity European (CE) Marking; Food and Drug Administration (FDA) Approval; Other Regulatory Certifications
5) By End-User: Medical Device Manufacturers; Regulatory Authorities; Healthcare Facilities; Independent Testing Laboratories
Subsegment:
1) By Active Medical Devices: Diagnostic Imaging Devices; Patient Monitoring Devices; Therapeutic Devices; Surgical Devices2) By Non-active Medical Devices: Surgical Instruments; Wound Care Products; Medical Consumables; Hospital Furniture and Equipment
3) By In-vitro Diagnostic Devices: Reagents and Kits; Analytical Instruments; Molecular Diagnostics Devices; Point-Of-Care Testing Devices
4) By Combination Products: Drug-Device Combination Products; Biologic-Device Combination Products; Device-Device Combination Products
Companies Mentioned: Société Générale de Surveillance S.A.; Eurofins Scientific S.E.; Bureau Veritas S.A.; Intertek Group plc; Charles River Laboratories International Inc.; TÜV SÜD Aktiengesellschaft; UL Solutions Inc.; Element Materials Technology Limited; QIMA Limited; Cotecna Inspection SA; North American Science Associates LLC; TÜV Rheinland AG; Freyr Solutions Pvt. Ltd.; Nelson Laboratories LLC; MED Institute Inc.; Auriga Research Limited; DDL Inc.; F2 Labs LLC; DeviceLab LLC; EdgeOne Medical Inc.
Countries: Australia; Brazil; China; France; Germany; India; Indonesia; Japan; Russia; South Korea; UK; USA; Canada; Italy; Spain.
Regions: Asia-Pacific; Western Europe; Eastern Europe; North America; South America; Middle East; Africa
Time Series: Five years historic and ten years forecast.
Data: Ratios of market size and growth to related markets, GDP proportions, expenditure per capita.
Data Segmentation: Country and regional historic and forecast data, market share of competitors, market segments.
Sourcing and Referencing: Data and analysis throughout the report is sourced using end notes.
Delivery Format: PDF, Word and Excel Data Dashboard.
Companies Mentioned
The companies featured in this Medical Device Testing, Inspection, and Certification market report include:- Société Générale de Surveillance S.A.
- Eurofins Scientific S.E.
- Bureau Veritas S.A.
- Intertek Group plc
- Charles River Laboratories International Inc.
- TÜV SÜD Aktiengesellschaft
- UL Solutions Inc.
- Element Materials Technology Limited
- QIMA Limited
- Cotecna Inspection SA
- North American Science Associates LLC
- TÜV Rheinland AG
- Freyr Solutions Pvt. Ltd.
- Nelson Laboratories LLC
- MED Institute Inc.
- Auriga Research Limited
- DDL Inc.
- F2 Labs LLC
- DeviceLab LLC
- EdgeOne Medical Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 250 |
| Published | November 2025 |
| Forecast Period | 2025 - 2029 |
| Estimated Market Value ( USD | $ 8.64 Billion |
| Forecasted Market Value ( USD | $ 11.81 Billion |
| Compound Annual Growth Rate | 8.1% |
| Regions Covered | Global |
| No. of Companies Mentioned | 20 |