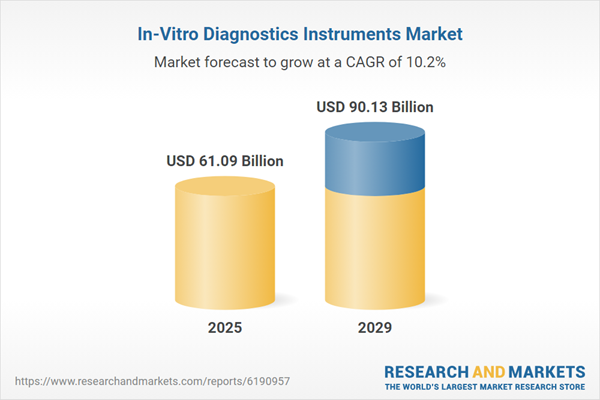
The in-vitro diagnostics instruments market size is expected to see rapid growth in the next few years. It will grow to $90.13 billion in 2029 at a compound annual growth rate (CAGR) of 10.2%. The growth during the forecast period is driven by the increasing demand for personalized medicine, growing demand for rapid diagnostic tests, rising demand for home-based diagnostics, and increasing awareness of antimicrobial resistance. Primary trends in the forecast period include the development of miniaturized diagnostic devices, advancement in high-throughput platforms, integration with electronic health records, incorporation of robotics in laboratories, and innovation in biosensor technologies.
The rising prevalence of chronic and lifestyle related diseases is expected to enhance the growth of the in vitro diagnostics instruments market in the coming years. Chronic and lifestyle related diseases refer to long term health conditions primarily influenced by daily habits, behaviors, and environmental factors, such as heart disease, diabetes, obesity, and certain cancers. The incidence of these diseases is increasing due to sedentary lifestyles and unhealthy dietary habits. In vitro diagnostics instruments are experiencing higher demand as the growing burden of chronic and lifestyle related diseases encourages companies to adopt early detection and monitoring tools for improved disease management. For example, in 2024, according to the American Action Forum, a U.S. based non profit, center right think tank, chronic diseases remain a major public health concern in the United States, with nearly 60 percent of adults living with at least one condition, 40 percent managing multiple conditions, and prevalence expected to nearly double by 2050. Furthermore, the chronic disease treatment market is projected to reach approximately 38.02 billion dollars by 2034, up from 9.74 billion dollars in 2025. Therefore, the rising prevalence of chronic and lifestyle related diseases is enhancing the growth of the in vitro diagnostics instruments market.
Prominent companies operating in the in-vitro diagnostics instruments market are focusing on developing advanced technologies, such as digital polymerase chain reaction (dPCR) systems, to achieve highly precise and sensitive nucleic acid quantification for next-generation diagnostics. Digital polymerase chain reaction (dPCR) systems are sophisticated diagnostic instruments that quantify DNA or RNA molecules by dividing samples into thousands of individual reactions, allowing for exceptional accuracy and sensitivity in detection. For example, in September 2024, QIAGEN N.V., a Netherlands-based biotechnology company, introduced the QIAcuityDx Digital PCR System, expanding its dPCR portfolio into clinical diagnostics with a primary focus on oncology testing. This fully integrated and automated system is specifically designed for clinical use, featuring a cartridge-based workflow that minimizes manual handling. Equipped with automated analysis and reporting software, the QIAcuityDx enables rapid and precise detection of genetic biomarkers for cancer diagnosis and therapy monitoring, reducing operator involvement while ensuring reliable and reproducible results.
In January 2025, Fremman Ltd., a UK-based investment company, acquired a majority stake in DIESSE Diagnostica Senese S.p.A. for an undisclosed amount. Through this acquisition, Fremman Ltd aims to expand DIESSE Diagnostica Senese's international presence, strengthen its leadership in specialty in vitro diagnostics, and support ongoing innovation to advance global diagnostic capabilities and improve patient outcomes. DIESSE Diagnostica Senese S.p.A. is an Italy-based medical equipment company that provides in vitro diagnostic instruments.
Major players operating in the in-vitro diagnostics instruments market are F. Hoffmann-La Roche Ltd, Danaher Corporation, Abbott Laboratories, Thermo Fisher Scientific, Siemens Healthineers AG, Illumina Inc., Hologic Inc., Qiagen N.V., Bio-Rad Laboratories Inc., Agilent Technologies, Becton, Dickinson and Company (BD), Sysmex Corporation, Laboratory Corporation of America Holdings (LabCorp), Guardant Health Inc., Quest Diagnostics Inc., Exact Sciences Corporation, Myriad Genetics Inc., Natera Inc., NeoGenomics Laboratories Inc., NanoString Technologies Inc., Veracyte Inc., Invitae Corporation, Foundation Medicine Inc. (Roche), Caris Life Sciences, Personalis Inc., OncoCyte Corporation, Freenome Holdings Inc., Epic Sciences Inc.
North America was the largest region in the in-vitro diagnostics instruments market in 2024. Asia-Pacific is expected to be the fastest-growing region in the forecast period. The regions covered in the in-vitro diagnostics instruments market report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East, Africa.
Note that the outlook for this market is being affected by rapid changes in trade relations and tariffs globally. The report will be updated prior to delivery to reflect the latest status, including revised forecasts and quantified impact analysis. The report’s Recommendations and Conclusions sections will be updated to give strategies for entities dealing with the fast-moving international environment.
The fast surge in U.S. tariffs and the trade tensions that followed in spring 2025 are heavily affecting the medical equipment sector, particularly for imported imaging machine components, surgical-grade stainless steel, and plastic disposables. Hospitals and clinics resist price hikes, pressuring manufacturers’ margins. Regulatory hurdles compound the problem, as tariff-related supplier changes often require re-certification of devices, delaying time-to-market. Companies are mitigating risks by dual-sourcing critical parts, expanding domestic production of commoditized items, and accelerating R&D in cost-efficient materials.
The in-vitro diagnostics instruments market research report is one of a series of new reports that provides in-vitro diagnostics instruments market statistics, including in-vitro diagnostics instruments industry global market size, regional shares, competitors with a in-vitro diagnostics instruments market share, detailed in-vitro diagnostics instruments market segments, market trends and opportunities, and any further data you may need to thrive in the in-vitro diagnostics instruments industry. This in-vitro diagnostics instruments market research report delivers a complete perspective of everything you need, with an in-depth analysis of the current and future scenario of the industry.
In-vitro diagnostic instruments are medical devices used to analyze biological samples, such as blood, urine, or tissue, outside the human body. Their primary function is to detect, diagnose, monitor, and manage diseases or medical conditions by providing accurate and timely laboratory test results. These instruments aid clinical decision-making and support personalized patient care.
The primary product types of in-vitro diagnostic instruments include clinical chemistry, immunoassay, molecular diagnostics, hematology, microbiology, coagulation, and others. Clinical chemistry instruments are digital tools, often leveraging AI, to analyze blood, urine, and other body fluids, helping identify biochemical abnormalities, improve diagnostic accuracy, and guide treatment planning. These instruments are applied across multiple areas, including infectious diseases, cancer, cardiology, nephrology, and autoimmune disorders. They serve a diverse range of end users, including hospitals, diagnostic laboratories, academic and research institutes, point-of-care facilities, blood banks, and pharmaceutical and biotechnology companies.
The countries covered in the in-vitro diagnostics instruments market report are Australia, Brazil, China, France, Germany, India, Indonesia, Japan, Russia, South Korea, UK, USA, Canada, Italy, Spain.
The in-vitro diagnostics instruments market consists of sales of point-of-care testing devices, flow cytometry instruments, histopathology and cytology instruments, and molecular point-of-care systems. Values in this market are ‘factory gate’ values, that is, the value of goods sold by the manufacturers or creators of the goods, whether to other entities (including downstream manufacturers, wholesalers, distributors, and retailers) or directly to end customers. The value of goods in this market includes related services sold by the creators of the goods.
The market value is defined as the revenues that enterprises gain from the sale of goods and/or services within the specified market and geography through sales, grants, or donations in terms of the currency (in USD unless otherwise specified).
The revenues for a specified geography are consumption values that are revenues generated by organizations in the specified geography within the market, irrespective of where they are produced. It does not include revenues from resales along the supply chain, either further along the supply chain or as part of other products.
This product will be delivered within 3-5 business days.
Table of Contents
Executive Summary
In-Vitro Diagnostics Instruments Global Market Report 2025 provides strategists, marketers and senior management with the critical information they need to assess the market.This report focuses on in-vitro diagnostics instruments market which is experiencing strong growth. The report gives a guide to the trends which will be shaping the market over the next ten years and beyond.
Reasons to Purchase:
- Gain a truly global perspective with the most comprehensive report available on this market covering 15 geographies.
- Assess the impact of key macro factors such as geopolitical conflicts, trade policies and tariffs, post-pandemic supply chain realignment, inflation and interest rate fluctuations, and evolving regulatory landscapes.
- Create regional and country strategies on the basis of local data and analysis.
- Identify growth segments for investment.
- Outperform competitors using forecast data and the drivers and trends shaping the market.
- Understand customers based on the latest market shares.
- Benchmark performance against key competitors.
- Suitable for supporting your internal and external presentations with reliable high quality data and analysis
- Report will be updated with the latest data and delivered to you along with an Excel data sheet for easy data extraction and analysis.
- All data from the report will also be delivered in an excel dashboard format.
Description
Where is the largest and fastest growing market for in-vitro diagnostics instruments? How does the market relate to the overall economy, demography and other similar markets? What forces will shape the market going forward, including technological disruption, regulatory shifts, and changing consumer preferences? The in-vitro diagnostics instruments market global report answers all these questions and many more.The report covers market characteristics, size and growth, segmentation, regional and country breakdowns, competitive landscape, market shares, trends and strategies for this market. It traces the market’s historic and forecast market growth by geography.
- The market characteristics section of the report defines and explains the market.
- The market size section gives the market size ($b) covering both the historic growth of the market, and forecasting its development.
- The forecasts are made after considering the major factors currently impacting the market. These include: the technological advancements such as AI and automation, Russia-Ukraine war, trade tariffs (government-imposed import/export duties), elevated inflation and interest rates.
- Market segmentations break down the market into sub markets.
- The regional and country breakdowns section gives an analysis of the market in each geography and the size of the market by geography and compares their historic and forecast growth.
- The competitive landscape chapter gives a description of the competitive nature of the market, market shares, and a description of the leading companies. Key financial deals which have shaped the market in recent years are identified.
- The trends and strategies section analyses the shape of the market as it emerges from the crisis and suggests how companies can grow as the market recovers.
Report Scope
Markets Covered:
1) By Product Type: Clinical Chemistry; Immunoassay; Molecular Diagnostics; Hematology; Microbiology; Coagulation; Other Product Types2) By Application: Infectious Diseases; Cancer; Cardiology; Nephrology; Autoimmune Diseases; Other Applications
3) By End User: Hospitals and Diagnostic Laboratories; Academic and Research Institutes; Point-Of-Care Settings; Blood Banks; Pharmaceutical and Biotechnology Companies; Other Healthcare Facilities
Subsegments:
1) By Clinical Chemistry: Automated Clinical Chemistry Analyzers; Semi-Automated Clinical Chemistry Analyzers; Point-Of-Care Clinical Chemistry Devices2) By Immunoassay: Enzyme-Linked Immunosorbent Assay (ELISA) Systems; Chemiluminescence Immunoassay (CLIA) Analyzers; Radioimmunoassay (RIA) Systems
3) By Molecular Diagnostics: Polymerase Chain Reaction (PCR) Systems; Next-Generation Sequencing (NGS) Platforms; Deoxyribonucleic Acid Microarrays; Isothermal Amplification Devices
4) By Hematology: Automated Hematology Analyzers; Manual Hematology Analyzers; Hemostasis Analyzers; Coagulation Analyzers
5) By Microbiology: Microbial Identification Systems; Antibiotic Susceptibility Testing (AST) Systems; Blood Culture Systems; Automated Microbial Analyzers.
6) By Coagulation: Coagulometers; Platelet Function Analyzers; Thromboelastography (TEG) Systems; Point-Of-Care Coagulation Devices
7) By Other Product Types: Point-Of-Care Diagnostic Devices; Clinical Chemistry Reagents; Diagnostic Imaging Instruments; Miscellaneous Laboratory Instruments
Companies Mentioned: F. Hoffmann-La Roche Ltd, Danaher Corporation, Abbott Laboratories, Thermo Fisher Scientific, Siemens Healthineers AG, Illumina Inc., Hologic Inc., Qiagen N.V., Bio-Rad Laboratories Inc., Agilent Technologies, Becton, Dickinson and Company (BD), Sysmex Corporation, Laboratory Corporation of America Holdings (LabCorp), Guardant Health Inc., Quest Diagnostics Inc., Exact Sciences Corporation, Myriad Genetics Inc., Natera Inc., NeoGenomics Laboratories Inc., NanoString Technologies Inc., Veracyte Inc., Invitae Corporation, Foundation Medicine Inc. (Roche), Caris Life Sciences, Personalis Inc., OncoCyte Corporation, Freenome Holdings Inc., Epic Sciences Inc.
Countries: Australia; Brazil; China; France; Germany; India; Indonesia; Japan; Russia; South Korea; UK; USA; Canada; Italy; Spain.
Regions: Asia-Pacific; Western Europe; Eastern Europe; North America; South America; Middle East; Africa
Time Series: Five years historic and ten years forecast.
Data: Ratios of market size and growth to related markets, GDP proportions, expenditure per capita.
Data Segmentation: Country and regional historic and forecast data, market share of competitors, market segments.
Sourcing and Referencing: Data and analysis throughout the report is sourced using end notes.
Delivery Format: PDF, Word and Excel Data Dashboard.
Companies Mentioned
The companies featured in this In-Vitro Diagnostics Instruments market report include:- F. Hoffmann-La Roche Ltd
- Danaher Corporation
- Abbott Laboratories
- Thermo Fisher Scientific
- Siemens Healthineers AG
- Illumina Inc.
- Hologic Inc.
- Qiagen N.V.
- Bio-Rad Laboratories Inc.
- Agilent Technologies
- Becton
- Dickinson and Company (BD)
- Sysmex Corporation
- Laboratory Corporation of America Holdings (LabCorp)
- Guardant Health Inc.
- Quest Diagnostics Inc.
- Exact Sciences Corporation
- Myriad Genetics Inc.
- Natera Inc.
- NeoGenomics Laboratories Inc.
- NanoString Technologies Inc.
- Veracyte Inc.
- Invitae Corporation
- Foundation Medicine Inc. (Roche)
- Caris Life Sciences
- Personalis Inc.
- OncoCyte Corporation
- Freenome Holdings Inc.
- Epic Sciences Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 250 |
| Published | November 2025 |
| Forecast Period | 2025 - 2029 |
| Estimated Market Value ( USD | $ 61.09 Billion |
| Forecasted Market Value ( USD | $ 90.13 Billion |
| Compound Annual Growth Rate | 10.2% |
| Regions Covered | Global |
| No. of Companies Mentioned | 30 |