Market expansion is fueled by a rising number of traumatic brain injuries, congenital skull abnormalities, and complex neurosurgical procedures. Continuous advancements in biomaterials, including bioresorbable polymers, titanium mesh, and customized 3D-printed implants, have significantly improved both functional and aesthetic outcomes after surgery. A cranial implant is a highly specialized device used in cranioplasty, a surgical procedure that restores or reconstructs skull defects. These implants serve multiple roles: they protect the brain in damaged or missing skull areas, restore the natural head contour, and, in some cases, improve neurological function. Depending on the clinical requirement, implants may be pre-manufactured or uniquely designed using 3D imaging and additive manufacturing to replicate a patient’s skull anatomy. The market is undergoing a technological transformation, shaped by innovations such as artificial intelligence (AI), 3D printing, and robotic-assisted systems that are enhancing precision, customization, and surgical outcomes.
The non-customized cranial implant segment generated USD 143.4 million in 2024 and is forecast to grow steadily at a CAGR of 5.7% throughout 2034. Non-customized implants continue to hold an essential place in cranial reconstruction surgeries, especially in situations where rapid response and cost-effective procedures are critical. Pre-fabricated implants are manufactured in standard dimensions and configurations, allowing surgeons to perform quick implantations during emergency or resource-limited operations, ensuring both efficiency and safety.
The traumatic brain injury segment held a 61.9% share in 2024. This category remains a major contributor to the overall market due to the high prevalence of trauma-related cranial defects and the increasing adoption of advanced implant solutions. Rising utilization of biocompatible materials such as titanium, PMMA, and PEEK for customized implants is enhancing patient recovery and surgical success rates. Designed through advanced imaging techniques, these implants help minimize risks like post-operative infections and cerebrospinal fluid leakage, while offering improved functional stability and cosmetic restoration.
Germany continues to lead the Europe Cranial Implant Market, demonstrating substantial growth potential driven by technological innovation and a mature healthcare ecosystem. The country’s advanced medical infrastructure, specialized neurosurgical capabilities, and rapidly aging demographic have made it a prime hub for cranial reconstruction procedures. German medical centers are increasingly adopting customized implant designs supported by cutting-edge imaging and digital design platforms. Furthermore, ongoing research in biomaterials and additive manufacturing is strengthening the production of patient-specific PEEK and titanium implants, improving surgical precision and long-term patient outcomes.
Key companies operating in the Europe Cranial Implant Market include Zimmer Biomet Holdings, Medtronic, Renishaw, Johnson & Johnson, Fin-ceramica Faenza S.p.A., 3di, Acumed LLC, Stryker Corporation, evonos GmbH & Co. KG, 3D Systems, KLS Martin Group, B. Braun SE, Integra LifeSciences Holdings Corporation, and Xilloc Medical Int B.V. To strengthen their position, companies in the Europe cranial implant industry are emphasizing innovation, collaboration, and product diversification. Many firms are investing in research and development to design next-generation implants that are lighter, stronger, and more biocompatible. Strategic partnerships with hospitals, surgical centers, and research institutions are enhancing access to clinical expertise and accelerating product validation.
Comprehensive Market Analysis and Forecast
- Industry trends, key growth drivers, challenges, future opportunities, and regulatory landscape
- Competitive landscape with Porter’s Five Forces and PESTEL analysis
- Market size, segmentation, and regional forecasts
- In-depth company profiles, business strategies, financial insights, and SWOT analysis
This product will be delivered within 2-4 business days.
Table of Contents
Companies Mentioned
The companies featured in this Europe Cranial Implant market report include:- 3di
- 3d Systems
- Acumed LLC
- B. Braun SE
- evonos GmbH & Co. KG
- Fin-ceramica Faenza S.p.A.
- Integra LifeSciences Holdings Corporation
- Johnson & Johnson
- KLS Martin Group
- Medtronic
- Renishaw
- Stryker Corporation
- Xilloc Medical Int B.V.
- Zimmer Biomet Holdings
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 90 |
| Published | November 2025 |
| Forecast Period | 2024 - 2034 |
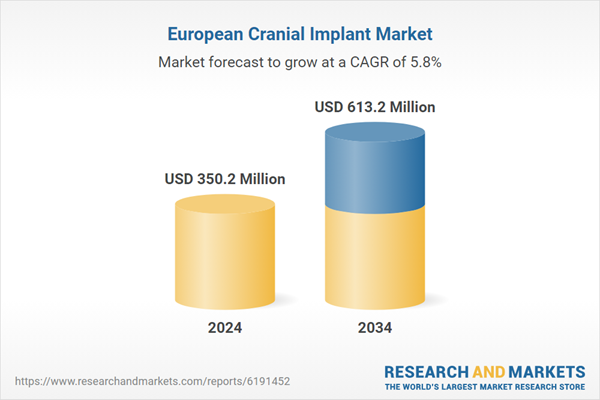
| Estimated Market Value ( USD | $ 350.2 Million |
| Forecasted Market Value ( USD | $ 613.2 Million |
| Compound Annual Growth Rate | 5.8% |
| Regions Covered | Europe |
| No. of Companies Mentioned | 15 |