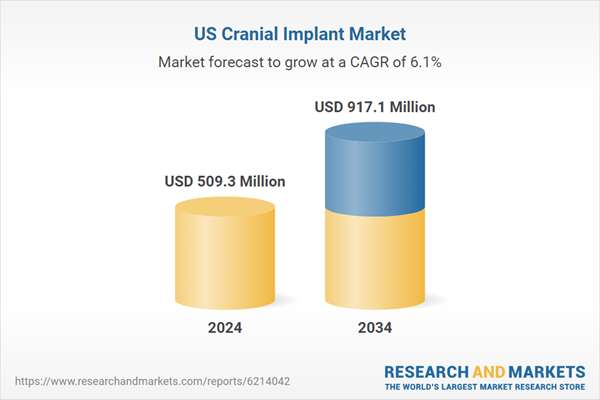
Growth in this field is supported by an increasing number of traumatic brain injuries, a strong clinical focus on reconstructive neurosurgery, and continuous advancements in personalized implant technologies. The United States holds one of the most advanced cranial implant landscapes, backed by a structured regulatory environment, well-developed surgical networks, and rapid adoption of patient-specific devices. Cranial implants play an essential role in procedures designed to replace or reinforce sections of the skull damaged by trauma, congenital abnormalities, tumors, or neurological surgeries. These implants are integral to restoring cranial structure, maintaining brain protection, and improving both functional and aesthetic outcomes for patients requiring complex reconstruction. As the number of individuals experiencing cranial injuries or postoperative defects increases, the requirement for durable, biocompatible materials continues to escalate. Healthcare providers are also shifting toward techniques that combine greater precision with faster surgical execution, which reinforces demand for advanced cranial implant solutions.
The non-customized category accounted for a 40.5% share in 2024. These standardized implants remain essential in U.S. trauma and emergency environments because they allow surgeons to act quickly without the wait times associated with custom preparation.
The metal implant segment was valued at 25.4% share in 2024 and continues to be widely selected for its mechanical strength, resilience, and reliability. Titanium-based devices are especially popular in cases that require maximum cranial stabilization and long-term durability.
The neurosurgery centers segment held a 26.6% share in 2024. These facilities specialize in neurological procedures and handle a high volume of reconstruction surgeries, contributing significantly to the procedural volume within the cranial implant industry.
Leading companies in the U.S. Cranial Implant Market include 3di, Medtronic, Matrix Surgical USA, Stryker Corporation, Renishaw, Zimmer Biomet Holdings, 3D Systems, KLS Martin Group, B. Braun SE, Johnson & Johnson, Acumed LLC, Integra LifeSciences Holdings Corporation, and Kelyniam Global. Companies operating in the U.S. Cranial Implant Market are advancing their market position by developing personalized implant solutions supported by 3D printing, image-guided modeling, and advanced biomaterials. Many firms are expanding their titanium and polymer implant portfolios to address both routine and complex reconstruction needs. Strategic partnerships with neurosurgery centers and hospitals help streamline surgeon training and improve clinical adoption. Manufacturers are also strengthening their regulatory compliance programs to accelerate product approvals and ensure consistent quality across customized and non-customized implants.
Comprehensive Market Analysis and Forecast
- Industry trends, key growth drivers, challenges, future opportunities, and regulatory landscape
- Competitive landscape with Porter’s Five Forces and PESTEL analysis
- Market size, segmentation, and regional forecasts
- In-depth company profiles, business strategies, financial insights, and SWOT analysis
This product will be delivered within 2-4 business days.
Table of Contents
Companies Mentioned
The companies profiled in this US Cranial Implant market report include:- 3di
- 3d Systems
- Acumed LLC
- B. Braun SE
- Integra LifeSciences Holdings Corporation
- Johnson & Johnson
- Kelyniam Global
- KLS Martin Group
- Matrix Surgical USA
- Medtronic
- Renishaw
- Stryker Corporation
- Zimmer Biomet Holdings
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 60 |
| Published | November 2025 |
| Forecast Period | 2024 - 2034 |
| Estimated Market Value ( USD | $ 509.3 Million |
| Forecasted Market Value ( USD | $ 917.1 Million |
| Compound Annual Growth Rate | 6.1% |
| Regions Covered | United States |
| No. of Companies Mentioned | 14 |