Other factors contributing to market growth include the growing adoption of digital compliance tools, such as RIM and eCTD, as well as market expansion that demands multi-region regulatory alignment and expertise. Such factors are expected to drive the market growth. The rapid expansion of research and development (R&D) activities in the U.S. pharmaceutical, biotechnology, and medical technology (MedTech) sectors is expected to boost the U.S. regulatory consulting outsourcing industry. The rising presence of pharmaceutical, biotechnology, and medical device companies has led to increased investments in innovative drugs, biologics, and advanced medical technologies, making regulatory submissions more complex. This surge in R&D initiatives has caused companies to depend more on consulting firms to navigate the evolving U.S. regulatory consulting outsourcing industry, thereby driving strong growth in outsourcing requirements and market revenue.
In addition, in the country, regulatory requirements have shifted from a primarily operational and compliance-driven discipline to a dynamic, strategic role that is critical to the success of pharmaceutical, biotechnological, and medical device development. This shift has increased the complexity of the regulatory landscape in the U.S. regulatory consulting outsourcing industry, which is expected to drive the requirement for regulatory consulting services.
Currently, the U.S. regulatory consulting outsourcing industry is becoming more fragmented with requirements at both federal and state levels. As a result, companies must comply with various Food and Drug Administration (FDA) divisions, such as those overseeing drugs, biologics, and medical devices, while also addressing evolving guidelines on labeling, advertising, and post-market surveillance. Furthermore, for most companies, maintaining in-house regulatory expertise across all product categories is becoming increasingly complex and costly, driving the need for outsourcing services.
Furthermore, the rapid growth of research and development (R&D) activities in the U.S. pharmaceutical, biotechnology, and medical technology (MedTech) sectors is expected to boost the expansion of the U.S. regulatory consulting outsourcing services industry. The increasing presence of pharmaceutical, biotechnology, and medical device companies has led to increased investments in innovative drugs, biologics, and advanced medical technologies, complicating regulatory submissions more complex. This surge in R&D initiatives has prompted companies to depend more on consulting firms to navigate the evolving U.S. regulatory landscape, thereby driving strong growth in outsourcing demand and market revenue. These factors are expected to drive the market growth over the estimated time period.
U.S. Regulatory Consulting Outsourcing Services Market Report Segmentation
This report forecasts revenue growth at the country level and provides an analysis of the latest industry trends in each sub-segment from 2021 to 2033. For this study, the analyst has segmented the U.S. regulatory consulting outsourcing services market report based on category, indication, end use, and company size.Category Outlook (Revenue, USD Million, 2021-2033)
- Drugs
- Biologics
- Medical Devices
Indication Outlook (Revenue, USD Million, 2021-2033)
- Oncology
- Neurology
- Cardiology
- Immunology
- Others
End Use Outlook (Revenue, USD Million, 2021-2033)
- Biotechnology Companies
- Pharmaceutical Companies
- Medical Device Companies
Company Size Outlook (Revenue, USD Million, 2021-2033)
- Small
- Medium
- Large
Why should you buy this report?
- Comprehensive Market Analysis: Gain detailed insights into the market across major regions and segments.
- Competitive Landscape: Explore the market presence of key players.
- Future Trends: Discover the pivotal trends and drivers shaping the future of the market.
- Actionable Recommendations: Utilize insights to uncover new revenue streams and guide strategic business decisions.
This report addresses:
- Market intelligence to enable effective decision-making
- Market estimates and forecasts from 2018 to 2030
- Growth opportunities and trend analyses
- Segment and regional revenue forecasts for market assessment
- Competition strategy and market share analysis
- Product innovation listings for you to stay ahead of the curve
Table of Contents
Companies Mentioned
The key companies profiled in this U.S. Regulatory Consulting Outsourcing Services market report include:- Parexel International Corporation
- ICON plc
- IQVIA
- Syneos Health
- ProPharma
- PharmaLex GmbH (Cencora)
- Freyr
- Lachman Consultants
- R&Q Solutions (RQM+)
- The FDA Group
- Emergo by UL
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 120 |
| Published | November 2025 |
| Forecast Period | 2024 - 2033 |
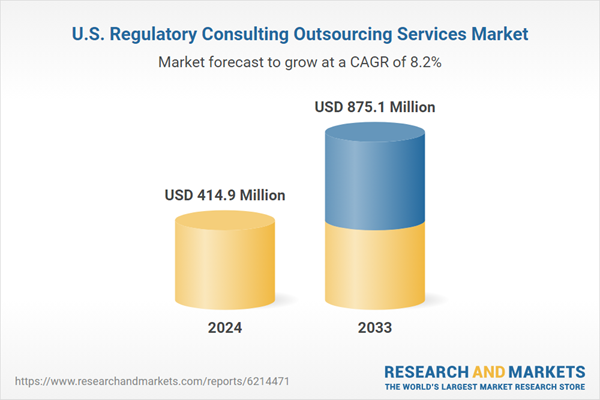
| Estimated Market Value ( USD | $ 414.9 Million |
| Forecasted Market Value ( USD | $ 875.1 Million |
| Compound Annual Growth Rate | 8.1% |
| Regions Covered | United States |
| No. of Companies Mentioned | 11 |