Speak directly to the analyst to clarify any post sales queries you may have.
10% Free customizationThis report comes with 10% free customization, enabling you to add data that meets your specific business needs.
Despite these favorable growth indicators, the market encounters significant obstacles related to the complex logistics required for vaccine distribution. Stringent cold chain storage necessities impose high operational costs and create distribution inefficiencies, particularly in developing nations that lack robust infrastructure. Consequently, these supply chain constraints constitute a substantial challenge that could limit the widespread accessibility of essential immunizations and hinder the overall expansion of the global travel vaccine sector.
Market Drivers
The revival of global international tourism and business travel serves as a major catalyst for market expansion, as heightened mobility directly leads to increased vaccine administration. As air connectivity improves and borders fully reopen, travelers are increasingly visiting exotic and tropical locations where endemic diseases present significant health risks, necessitating prophylactic immunization. This upward trend in mobility secures a sustained patient base for travel medicine clinics and pharmaceutical manufacturers, particularly for vaccines protecting against hepatitis A, typhoid, and yellow fever. Highlighting this recovery, the International Air Transport Association's 'Air Passenger Market Analysis' from July 2024 noted that total international passenger demand increased by 14.6 percent in May 2024 compared to the previous year, underscoring the rapid restoration of cross-border movement that supports market demand.Simultaneously, the rising prevalence of emerging and re-emerging infectious diseases in popular tourist destinations compels travelers to obtain vaccinations before departure. Outbreaks of vector-borne illnesses in tropical regions have increased the urgency for preventative measures among tourists and business expatriates, driving the adoption of newly approved immunization options.
For example, the significant expansion of dengue fever transmission zones has created an acute demand for preventative solutions. The Pan American Health Organization reported in its March 2024 'Epidemiological Update Dengue' that the Americas region saw over 3.5 million suspected dengue cases in early 2024, a staggering rise compared to historical averages. This epidemiological landscape generates tangible financial gains for vaccine developers; Bavarian Nordic reported that its Travel Health segment achieved revenues of DKK 1.87 billion for the full year of 2023, reflecting the robust commercial impact of high infection risks.
Market Challenges
Complex logistics and stringent cold chain storage requirements act as a formidable barrier to the growth of the Global Travel Vaccine Market. Travel vaccines are biological products that typically require precise temperature controls, ranging from refrigerated to cryogenic states, throughout their entire transit. in regions with underdeveloped infrastructure, maintaining this thermal continuity is both operationally difficult and financially burdensome. The resulting high operational costs and elevated risks of product spoilage deter manufacturers and distributors from efficiently expanding into these markets. Consequently, this logistical fragility effectively places a ceiling on revenue potential, as the industry struggles to guarantee the reliable availability of essential immunizations in destinations where they are most needed.This operational strain is further intensified by the immense volume of products that must navigate these often-constrained supply networks. The pressure to transport vast quantities of temperature-sensitive vials amplifies existing distribution inefficiencies. According to UNICEF's Supply Annual Report 2024, the organization managed the international delivery of 2.787 billion vaccine doses to 99 countries in 2024. Processing such a massive volume through fragile cold chains inevitably leads to bottlenecks and supply interruptions. These constraints directly restrict market reach and undermine the industry's capacity to fully capitalize on the heightened demand for prophylactic healthcare driven by the resurgence of global tourism.
Market Trends
The commercialization of novel vaccines for vector-borne diseases is shifting the market from research to the availability of approved prophylactics for neglected tropical illnesses. Developers are navigating regulatory pathways to launch immunizations for diseases like chikungunya, filling the preventative care gap for travelers visiting endemic regions. This transition from pipeline to product generates immediate commercial value and expands the travel health portfolio beyond traditional vaccines. According to Bavarian Nordic A/S, in its November 2025 'Interim Results for the First Nine Months of 2025', Travel Health revenue increased by 23 percent to DKK 2.32 billion, a growth trajectory supported by the commercial launch of its chikungunya vaccine, Vimkunya.Concurrently, the development of multivalent and combination vaccine formulations is reshaping immunization strategies by offering broad-spectrum protection through single-product solutions. Manufacturers are prioritizing tetravalent technologies that induce immunity against multiple virus serotypes simultaneously, simplifying vaccination schedules and enhancing traveler compliance compared to monovalent alternatives. This innovation improves clinical outcomes and drives market uptake by streamlining the logistical burden for travel medicine providers. According to Takeda Pharmaceutical Company Limited's January 2025 'earnings results for the third quarter of fiscal year 2024', the company reported that its tetravalent dengue vaccine, Qdenga, generated 19.9 billion JPY in revenue for the first half of the fiscal year, marking an 863 percent increase.
Key Players Profiled in the Travel Vaccine Market
- GlaxoSmithKline, PLC
- Sanofi S.A.
- Novartis International AG
- Merck KGaA
- Pfizer Inc.
- AstraZeneca PLC
- Abbott Laboratories, Inc.
- F. Hoffmann-La Roche AG
- CSL Limited
- Johnson & Johnson
Report Scope
In this report, the Global Travel Vaccine Market has been segmented into the following categories:Travel Vaccine Market, by Composition:
- Mono Vaccines v/s Combination Vaccines
Travel Vaccine Market, by Disease Indication:
- Hepatitis A
- DPT
- Yellow Fever
- Typhoid
- Hepatitis B
- Measles and Mumps
- Rabies
- Meningococcal
- Varicella
- Japanese Encephalitis
- Others
Travel Vaccine Market, by Travel Type:
- Domestic v/s International
Travel Vaccine Market, by Region:
- North America
- Europe
- Asia-Pacific
- South America
- Middle East & Africa
Competitive Landscape
Company Profiles: Detailed analysis of the major companies present in the Global Travel Vaccine Market.Available Customization
The analyst offers customization according to your specific needs. The following customization options are available for the report:- Detailed analysis and profiling of additional market players (up to five).
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
The key players profiled in this Travel Vaccine market report include:- GlaxoSmithKline, PLC
- Sanofi S.A.
- Novartis International AG
- Merck KGaA
- Pfizer Inc.
- AstraZeneca PLC
- Abbott Laboratories, Inc
- F. Hoffmann-La Roche AG
- CSL Limited
- Johnson & Johnson
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 182 |
| Published | January 2026 |
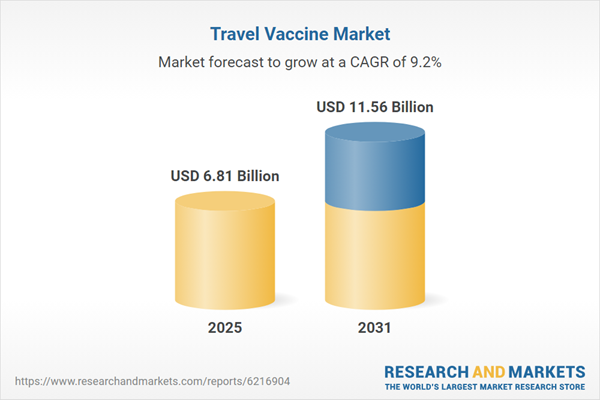
| Forecast Period | 2025 - 2031 |
| Estimated Market Value ( USD | $ 6.81 Billion |
| Forecasted Market Value ( USD | $ 11.56 Billion |
| Compound Annual Growth Rate | 9.2% |
| Regions Covered | Global |
| No. of Companies Mentioned | 11 |