Speak directly to the analyst to clarify any post sales queries you may have.
10% Free customizationThis report comes with 10% free customization, enabling you to add data that meets your specific business needs.
Despite these growth prospects, the market faces a significant hurdle due to the high costs and complexity involved in managing the rapidly increasing volume of safety data. This surge in data places immense pressure on existing resources and necessitates the development of robust infrastructure. For instance, the European Medicines Agency reported that in 2024, over 1.7 million adverse drug reaction reports were submitted to EudraVigilance, underscoring the massive scale of information that organizations must accurately capture and analyze to ensure regulatory compliance.
Market Drivers
The escalating prevalence of chronic diseases and the resulting increase in medication consumption serve as key catalysts for the Global Pharmacovigilance Market. As the global burden of conditions like oncology, diabetes, and cardiovascular disorders rises, the volume of administered drugs increases, necessitating strict monitoring for adverse events. Highlighting this trend, the World Health Organization's 'Global Status Report on Cancer 2024', released in February 2024, estimated there were 20 million new cancer cases in 2022, indicating a growing patient population that requires long-term pharmacological treatment. This rise in disease incidence compels pharmaceutical entities to expand their pharmacovigilance operations to effectively track the safety profiles of complex therapies used in chronic care.Furthermore, the expansion of clinical trials and drug research and development accelerates market growth by generating vast amounts of safety data that must be processed before authorization. Pharmaceutical companies are investing heavily in innovation to meet unmet medical needs, heightening the demand for surveillance during both clinical and post-marketing phases. According to the European Federation of Pharmaceutical Industries and Associations' June 2024 report, 'The Pharmaceutical Industry in Figures 2024', R&D expenditure in Europe was projected to reach €50.00 billion in 2023. To manage the regulatory oversight associated with this investment, agencies are also enhancing their capabilities; the US Food and Drug Administration requested a budget of $7.2 billion for fiscal year 2025 in 2024 to strengthen safety programs and public health infrastructure.
Market Challenges
The rapid increase in safety data volume poses a significant barrier to the Global Pharmacovigilance Market's expansion. As adverse event reports multiply exponentially, pharmaceutical companies are forced to divert substantial capital toward maintaining basic compliance rather than investing in innovation. The complexity and high expense of validating and processing this influx create a severe financial strain, particularly for small and medium-sized enterprises. These smaller entities often lack the budgetary flexibility to absorb the soaring operational costs required for rigorous safety surveillance, effectively limiting their participation in the market and hampering overall industry growth.This resource-intensive environment is further exacerbated by the sheer scale of information that must be managed to avoid regulatory penalties. The relentless acceleration of data intake compels organizations to prioritize immediate processing capabilities over long-term strategic development. According to the Uppsala Monitoring Centre, the global VigiBase system received an average of approximately 50,000 new individual case safety reports per week in 2024. This magnitude of data influx highlights the immense pressure on industry stakeholders, who must continuously expand their infrastructure solely to keep pace with reporting requirements, thereby stifling broader market opportunities and profitability.
Market Trends
The integration of Artificial Intelligence and Machine Learning is fundamentally reshaping the Global Pharmacovigilance Market by shifting safety operations from reactive compliance to proactive risk management. Facing an exponential rise in adverse event reports, pharmaceutical companies are increasingly utilizing predictive algorithms and natural language processing to automate labor-intensive tasks such as case intake, validity checks, and narrative generation. This technological advancement allows safety teams to focus on complex benefit-risk assessments rather than manual data entry, improving the speed and accuracy of signal detection. According to the Pistoia Alliance's 'Lab of the Future 2025 Report' from September 2025, 60% of respondents identified AI and machine learning as their top technology investment for the next two years, underscoring the sector's commitment to digital transformation.Simultaneously, strategic outsourcing to Contract Research Organizations (CROs) has emerged as a dominant trend, driven by the need to navigate complex regulatory landscapes and manage fluctuating resource demands without incurring fixed overheads. Small and medium-sized enterprises, in particular, are leveraging the specialized infrastructure of CROs to ensure global compliance and scalability, effectively converting fixed operational costs into variable expenses. This reliance on external partners enables bio-pharmaceutical firms to access advanced vigilance platforms and global safety networks that would be prohibitively expensive to build in-house. As reported by the Association of Clinical Research Organizations in July 2025, member companies generated an estimated $98 billion in revenue in 2024, reflecting the substantial scale of operations now entrusted to third-party providers.
Key Players Profiled in the Pharmacovigilance Market
- IQVIA Inc.
- ArisGlobal
- Parexel International Corporation
- Labcorp Drug Development
- Accenture
- Cognizant Technology Solutions
- Ergomed Group
- ICON PLC
- Syneos Health
- Wipro Limited
Report Scope
In this report, the Global Pharmacovigilance Market has been segmented into the following categories:Pharmacovigilance Market, by Clinical Trial Phase:
- Pre-Clinical
- Phase 1
- Phase 2
- Phase 3
- Phase 4
Pharmacovigilance Market, by Method:
- Spontaneous Reporting
- Intensified ADR Reporting
- Targeted Spontaneous Reporting
- Cohort Event Monitoring
- EHR Mining
Pharmacovigilance Market, by Service Provider:
- In-House
- Contract Outsourcing
Pharmacovigilance Market, by Process Flow:
- Case Data Management
- Signal Detection
- Risk Management System
Pharmacovigilance Market, by Therapeutic Area:
- Oncology
- Neurology
- Cardiology
- Others
Pharmacovigilance Market, by End-User:
- Pharmaceutical & Biotechnology Companies
- Medical Device Manufacturers
- Others
Pharmacovigilance Market, by Region:
- North America
- Europe
- Asia-Pacific
- South America
- Middle East & Africa
Competitive Landscape
Company Profiles: Detailed analysis of the major companies present in the Global Pharmacovigilance Market.Available Customization
The analyst offers customization according to your specific needs. The following customization options are available for the report:- Detailed analysis and profiling of additional market players (up to five).
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
The key players profiled in this Pharmacovigilance market report include:- IQVIA Inc.
- ArisGlobal
- Parexel International Corporation
- Labcorp Drug Development
- Accenture
- Cognizant Technology Solutions
- Ergomed Group
- ICON PLC
- Syneos Health
- Wipro Limited
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 185 |
| Published | January 2026 |
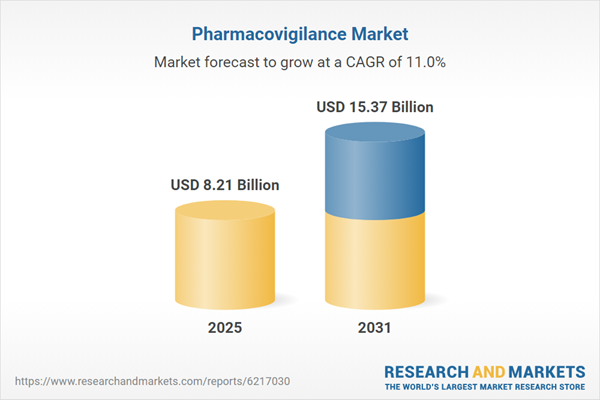
| Forecast Period | 2025 - 2031 |
| Estimated Market Value ( USD | $ 8.21 Billion |
| Forecasted Market Value ( USD | $ 15.37 Billion |
| Compound Annual Growth Rate | 11.0% |
| Regions Covered | Global |
| No. of Companies Mentioned | 11 |