Speak directly to the analyst to clarify any post sales queries you may have.
10% Free customizationThis report comes with 10% free customization, enabling you to add data that meets your specific business needs.
According to 2024 data from the Epilepsy Foundation, approximately 20% of children diagnosed with Landau-Kleffner Syndrome carry a mutation in the GRIN2A gene, highlighting the urgent need for precision medicine strategies tailored to specific genetic etiologies. A major obstacle hindering market growth is the frequent misidentification of the condition as autism or hearing loss, which results in considerable delays in administering necessary medical interventions and adversely affects long-term treatment outcomes.
Market Drivers
Increasing research and development investment in rare pediatric neurology is fundamentally transforming the Global LKS Treatment Market, shifting the focus from broad-spectrum anticonvulsants to disease-modifying therapies. This influx of capital is dedicated to understanding the complex genetic foundations of developmental epileptic encephalopathies, enabling the creation of treatments that address root causes rather than merely managing symptoms. The economic rationale for this increased spending is supported by the high cost of the condition; the National Institutes of Health reported in 2024 that annual direct medical costs for uncontrolled epilepsy reached approximately $30,343, emphasizing the commercial viability of effective interventions. Consequently, organizations like the Epilepsy Research Institute are scaling up funding, having invested over £2 million in research grants in their 2023-24 Annual Review to accelerate clinical innovation.The expansion of orphan drug designations and regulatory incentives acts as a second pivotal driver, mitigating the commercial risks associated with developing therapies for small patient populations like those with Landau-Kleffner Syndrome. Regulatory bodies are cultivating a favorable environment for pharmaceutical companies by offering benefits such as tax credits, fee waivers, and extended market exclusivity. This supportive framework is yielding tangible progress in the pipeline for rare genetic conditions. For instance, Global Genes reported in October 2024 that the FDA granted Rare Pediatric Disease designations to five experimental gene therapies under the Bespoke Gene Therapy Consortium, milestones that not only validate emerging candidates but also attract further biotechnology investment into the LKS sector.
Market Challenges
The frequent misdiagnosis of Landau-Kleffner Syndrome serves as a substantial impediment to the growth of the Global LKS Treatment Market. Because the disorder presents with symptoms mimicking autism spectrum disorder and hearing impairments, many pediatric patients undergo prolonged periods of ineffective management before receiving an accurate evaluation. This diagnostic confusion directly hampers market expansion by leaving a significant portion of the addressable patient population untreated or incorrectly medicated, thereby reducing the immediate commercial demand for specific anticonvulsant and corticosteroid therapies.Consequently, pharmaceutical manufacturers face a restricted revenue base as potential consumers remain unidentified during the critical early stages of the disease. According to a major 2024 survey by Rare Diseases International regarding diagnostic journeys, 60% of individuals with rare diseases were initially misdiagnosed with a different physical or psychological condition. This high rate of error in the broader rare disease landscape suggests that the specific market for LKS treatments is likely operating well below its true potential capacity due to these systemic identification failures.
Market Trends
The adoption of tele-rehabilitation platforms for speech therapy is emerging as a critical trend in the Global LKS Treatment Market, addressing the urgent need for consistent linguistic intervention in patients with acquired aphasia. This shift helps overcome the scarcity of specialized speech-language pathologists in remote areas, allowing for the high-frequency therapy sessions essential for regaining language skills that are often logistically challenging with traditional in-person visits. The integration of digital health tools facilitates continuous monitoring of cognitive progress and ensures adherence to rehabilitation protocols, a trend supported by the American Medical Association's December 2025 report, which noted that 32.2% of neurologists conducted more than 20% of their weekly patient visits via telehealth.Concurrently, the rising utilization of Intravenous Immunoglobulin (IVIG) therapies reflects a clinical pivot towards aggressive immunomodulation to manage the epileptic and cognitive regression associated with the syndrome. As evidence regarding the autoimmune etiology of Landau-Kleffner Syndrome grows, clinicians are increasingly prescribing IVIG for cases refractory to standard anticonvulsants and corticosteroids to halt the progression of continuous spike-and-wave during sleep (CSWS). This trend is substantially increasing the commercial demand for plasma-derived therapies within the broader neurology sector; for example, Takeda Pharmaceutical Company Limited reported in May 2025 that revenue for its immunoglobulin product portfolio grew by 17.6% year-over-year, underscoring the expanding global reliance on these immune-targeting interventions.
Key Players Profiled in the LKS Treatment Market
- Johnson & Johnson
- Novartis AG
- Pfizer Inc.
- Teva Pharmaceutical Industries Ltd.
- Abbott Laboratories Inc.
- Cipla Inc.
- Glenmark Pharmaceuticals Limited
- Mankind Pharma Limited
- Novo Nordisk A/S
- Takeda Pharmaceutical Company Limited
Report Scope
In this report, the Global LKS Treatment Market has been segmented into the following categories:LKS Treatment Market, by Type:
- Focal Motor Seizures
- Tonic Seizures
- Atonic Seizures
LKS Treatment Market, by Treatment:
- Anticonvulsant Drugs
- Corticosteroids
- Intravenous Immunoglobulins
- Surgery
- Speech Therapy
- Others
LKS Treatment Market, by Diagnosis:
- Electroencephalogram (EEG)
- MRI
- Audiometry
- Genetic Testing
- Others
LKS Treatment Market, by End User:
- Hospitals & Clinics
- Ambulatory Care Centers
- Others
LKS Treatment Market, by Region:
- North America
- Europe
- Asia-Pacific
- South America
- Middle East & Africa
Competitive Landscape
Company Profiles: Detailed analysis of the major companies present in the Global LKS Treatment Market.Available Customization
The analyst offers customization according to your specific needs. The following customization options are available for the report:- Detailed analysis and profiling of additional market players (up to five).
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
The key players profiled in this LKS Treatment market report include:- Johnson & Johnson
- Novartis AG
- Pfizer Inc.
- Teva Pharmaceutical Industries Ltd
- Abbott Laboratories Inc.
- Cipla Inc.
- Glenmark Pharmaceuticals Limited
- Mankind Pharma Limited
- Novo Nordisk A/S
- Takeda Pharmaceutical Company Limited
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 182 |
| Published | January 2026 |
| Forecast Period | 2025 - 2031 |
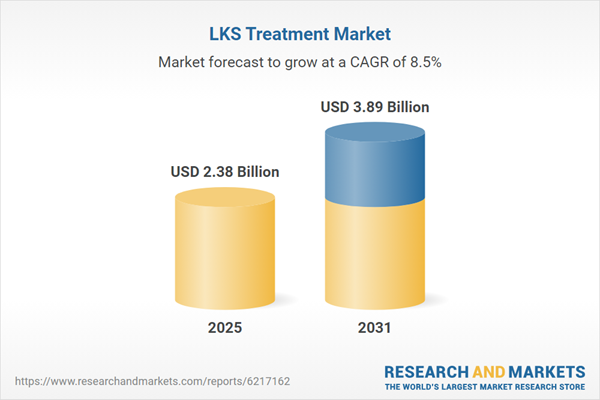
| Estimated Market Value ( USD | $ 2.38 Billion |
| Forecasted Market Value ( USD | $ 3.89 Billion |
| Compound Annual Growth Rate | 8.5% |
| Regions Covered | Global |
| No. of Companies Mentioned | 11 |