Speak directly to the analyst to clarify any post sales queries you may have.
10% Free customizationThis report comes with 10% free customization, enabling you to add data that meets your specific business needs.
However, the market faces substantial hurdles regarding strict regulatory compliance and quality assurance. Regulatory authorities mandate rigorous sterility and safety protocols for ophthalmic products, requiring intricate manufacturing systems and extensive clinical validation prior to market approval. Consequently, manufacturers contend with high compliance costs and the inherent risk of product recalls, which can disrupt supply chains and damage consumer trust. These regulatory challenges serve as a significant restraint, potentially slowing the introduction of new formulations to patients and limiting the overall expansion of the sector.
Market Drivers
The rising incidence of digital eye strain and dry eye syndrome acts as a primary catalyst for the Global Eye Drop market. Modern lifestyles involving prolonged exposure to digital screens have triggered a sharp increase in ocular surface diseases, necessitating the frequent use of artificial tears and lubricating drops. This trend is further intensified by environmental stressors and the growing use of contact lenses, which contribute to tear film instability. Manufacturers are meeting this need with targeted therapies, as reflected in strong financial results; for instance, Bausch + Lomb’s February 2025 "Fourth-Quarter and Full-Year 2024 Results" announcement indicated that their dry eye portfolio revenue is nearing $1 billion, highlighting the urgent consumer demand for effective symptom relief.Simultaneously, the increasing prevalence of chronic ophthalmic disorders, especially within the aging global population, significantly fuels industry growth. As demographic trends shift toward an older society, incidence rates of age-related conditions such as glaucoma and cataracts rise, creating a continuous demand for prescription eye drops for intraocular pressure management and post-surgical care. This ensures a stable patient base for therapeutic solutions. According to Alcon’s "2024 Annual Report" from February 2025, the Vision Care franchise generated approximately $4.3 billion in net sales. Furthermore, Vision Monday reported in February 2025 that Bausch + Lomb achieved total revenue of $4.79 billion, underscoring the enduring necessity for comprehensive eye health products.
Market Challenges
Stringent regulatory compliance and quality assurance protocols constitute a major impediment to the advancement of the global eye drop industry. Given that these products are administered to the sensitive ocular surface, regulators enforce sterility standards comparable to those for injectable medications. This necessitates that manufacturers maintain complex, capital-intensive production environments and navigate protracted clinical validation procedures. These high barriers to entry and operation inevitably inflate development costs, decelerate product innovation, and delay the commercialization of new therapeutic options.Failure to strictly adhere to these precise standards often leads to regulatory interventions that interrupt the supply chain. Manufacturing inconsistencies can result in product recalls and production stoppages, causing significant gaps in market availability. According to the American Society of Health-System Pharmacists, a record 323 active drug shortages were reported in 2024, with sterile ophthalmic preparations frequently cited as a vulnerable category due to ongoing quality management difficulties. These supply constraints directly reduce market volume and revenue while undermining the confidence of prescribers and patients, thereby limiting the sector's broader growth potential.
Market Trends
The widespread adoption of multi-dose preservative-free packaging technologies is reshaping manufacturing as companies prioritize ocular surface health. Traditional formulations often utilize preservatives like benzalkonium chloride that can damage the epithelium, prompting a transition toward complex dispensing systems that ensure sterility without additives. This shift effectively combines the convenience of bottled eye drops with the safety profile of unit-dose vials. According to Aptar Pharma’s February 2025 "Fourth Quarter and Annual 2024 Results," the segment responsible for proprietary drug delivery systems, including ophthalmic squeeze dispensers, achieved 9% sales growth for the full year 2024, reflecting the industrial surge toward these advanced container closure systems.Concurrently, the emergence of lipid-based and nano-emulsion formulations is transforming the treatment of evaporative dry eye and Meibomian Gland Dysfunction. Unlike standard aqueous solutions that only replenish the water layer, these innovative therapies target the tear film's lipid layer to prevent evaporation, addressing the root cause of most dry eye cases. This specific therapeutic focus has gained rapid commercial traction as manufacturers launch water-free and lipid-enhanced solutions. For example, Bausch + Lomb’s February 2025 "Fourth-Quarter and Full-Year 2024 Results" announcement reported approximately $53 million in fourth-quarter sales for Miebo, a novel lipid-based prescription drop, highlighting the immediate market uptake of treatments addressing tear evaporation.
Key Players Profiled in the Eye Drop market
- Johnson & Johnson Inc.
- Genentech, Inc.
- Alcon Inc.
- Pfizer, Inc.
- Bausch Health Companies, Inc.
- Bayer Corporation
- Allergan, Inc.
- Abbott Laboratories, Inc.
- KC Pharmaceuticals, Inc.
- Akorn, Inc.
Report Scope
In this report, the Global Eye Drop market has been segmented into the following categories:Eye Drop market, by Type:
- Prescription v/s Over-the-Counter
Eye Drop market, by Drug Class:
- Anti-allergy
- Anti-glaucoma
- Anti-inflammatory
- Anti-VEGF
- Others
Eye Drop market, by Disease Indication:
- Eye Allergy
- Glaucoma
- Eye Infections
- Dry Eye Diseases
- Retinal Disorders
Eye Drop market, by Distribution Channel:
- Hospitals Pharmacy
- Retail Pharmacy
- Online Pharmacy
Eye Drop market, by Region:
- North America
- Europe
- Asia-Pacific
- South America
- Middle East & Africa
Competitive Landscape
Company Profiles: Detailed analysis of the major companies present in the Global Eye Drop market.Available Customization
The analyst offers customization according to your specific needs. The following customization options are available for the report:- Detailed analysis and profiling of additional market players (up to five).
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
The key players profiled in this Eye Drop market report include:- Johnson & Johnson Inc.
- Genentech, Inc.
- Alcon Inc.
- Pfizer, Inc.
- Bausch Health Companies, Inc.
- Bayer Corporation
- Allergan, Inc.
- Abbott Laboratories, Inc.
- KC Pharmaceuticals, Inc.
- Akorn, Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 185 |
| Published | January 2026 |
| Forecast Period | 2025 - 2031 |
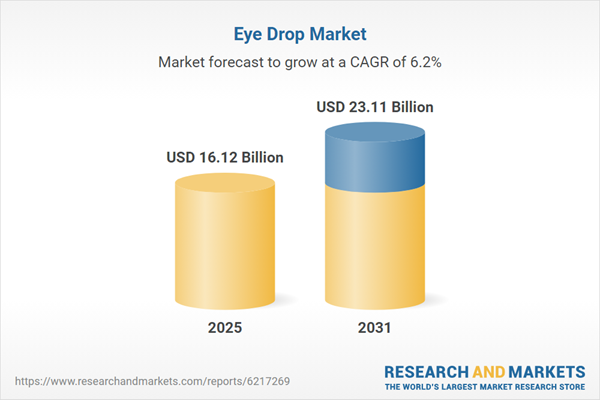
| Estimated Market Value ( USD | $ 16.12 Billion |
| Forecasted Market Value ( USD | $ 23.11 Billion |
| Compound Annual Growth Rate | 6.1% |
| Regions Covered | Global |
| No. of Companies Mentioned | 11 |