Speak directly to the analyst to clarify any post sales queries you may have.
10% Free customizationThis report comes with 10% free customization, enabling you to add data that meets your specific business needs.
However, the market faces a substantial hurdle in the form of rigorous regulatory compliance standards for medical devices, which extends product development timelines and inflates costs for both producers and end-users. This regulatory burden frequently postpones the arrival of innovative technologies in regions where they are most critically needed. The urgency for these health and environmental monitoring solutions is underscored by recent data; according to the American Lung Association's 2024 report, 131.2 million individuals in the United States resided in locations plagued by unhealthy air pollution levels.
Market Drivers
The escalating global incidence of asthma and respiratory disorders acts as a major driver for the broad adoption of asthma warning sensors. As diagnosis rates climb internationally, health systems are under mounting pressure to deploy effective management strategies that avert severe flare-ups and hospital stays, leading to higher demand for continuous monitoring devices with early warning features.For example, the World Health Organization highlighted in May 2024 that asthma impacts roughly 262 million individuals globally, establishing a massive market for these technologies. Additionally, the significant financial strain of untreated conditions drives the need for cost-efficient solutions; the Asthma and Allergy Foundation of America reported in March 2024 that asthma's annual economic cost to the U.S. surpassed $82 billion, highlighting the financial necessity for advanced preventive tools.
Rapid progress in IoT integration and wearable sensor technology is reshaping the market by upgrading device capabilities and boosting user adherence. Contemporary sensors now frequently track medication use in real-time and synchronize seamlessly with mobile apps, enabling patients and clinicians to monitor environmental triggers and treatment success. These technical enhancements support remote monitoring and significantly better clinical results by promoting strict medication adherence and facilitating prompt care. A 2024 study in the Journal of Asthma revealed that severe asthma patients using smart inhaler technology saw marked improvements in control, with Asthma Control Questionnaire (ACQ-6) scores dropping from a baseline of 2.81 to 1.92 at follow-up, a proven utility that accelerates the adoption of connected devices in standard respiratory protocols.
Market Challenges
Rigorous regulatory compliance represents a significant obstacle to the growth of the Global Asthma Warning Sensors Market. Because these devices increasingly rely on clinical-grade monitoring and predictive algorithms, they are often categorized as medical devices rather than standard consumer electronics, subjecting them to stringent safety validation, testing, and data privacy mandates from global health authorities. The substantial resources needed to manage these complicated approval processes force manufacturers to extend development timelines, effectively delaying the commercial release of cutting-edge sensing technologies. Consequently, these high entry barriers deter smaller innovators and reduce the overall speed of technological progress within the industry.The operational and financial pressure caused by these regulatory requirements is both severe and measurable. Manufacturers are compelled to direct substantial capital toward certification and compliance, diverting funds that could otherwise support research and development, making the cost of launching new sensors prohibitive. According to MedTech Europe, certification and maintenance expenses for medical devices rose by up to 100% in 2024 compared to earlier directives, resulting in a 33% reduction in major manufacturers choosing the region for initial product launches. These increased costs and subsequent delays restrict patient access to essential asthma management tools and hinder the market's capacity to react quickly to worsening environmental factors.
Market Trends
The integration of AI-powered predictive analytics is transforming the Global Asthma Warning Sensors Market from reactive alerting systems into proactive forecasting tools. By analyzing complex data sources like environmental variables and electronic health records, advanced algorithms can detect the risk of exacerbations well before physical symptoms manifest. This shift enables healthcare providers to execute early interventions that avert severe attacks, thereby optimizing health system resource usage. Recent research validates the clinical power of these models; a May 2025 study in EClinicalMedicine noted that a new AI-based passive digital marker achieved a prognostic accuracy Area Under the Curve (AUC) of 0.79, significantly surpassing traditional risk assessment methods.Concurrently, the rise of smartphone-based acoustic wheeze analysis is broadening market reach by using standard mobile microphones for objective assessment, removing the need for dedicated hardware. This technology evaluates respiratory sounds and vocal biomarkers to identify airway obstruction, providing patients with a convenient, non-invasive substitute for peak flow meters or spirometry. The capacity to conduct clinical-grade assessments via common consumer electronics lowers entry barriers for sophisticated monitoring solutions. The diagnostic strength of these acoustic tools is backed by robust data; a study published in Healthcare in March 2025 reported that a machine learning model analyzing specific speech phonemes achieved a classification accuracy of 98.7% in determining asthma status.
Key Players Profiled in the Asthma Warning Sensors Market
- Philips Healthcare
- ResMed Inc.
- Teva Pharmaceutical Industries Ltd.
- GlaxoSmithKline PLC
- Novartis AG
- AstraZeneca PLC
- Merck & Co., Inc.
- Propeller Health
- Adherium Limited
- Cohero Health, Inc.
Report Scope
In this report, the Global Asthma Warning Sensors Market has been segmented into the following categories:Asthma Warning Sensors Market, by Type:
- Wearable v/s Non-Wearable
Asthma Warning Sensors Market, by Technology:
- AI
- IoT
- Machine Learning
- Others
Asthma Warning Sensors Market, by Parameter Measured:
- FEV1 v/s Peak Flow
Asthma Warning Sensors Market, by End User:
- Hospitals & Clinics v/s Homecare
Asthma Warning Sensors Market, by Region:
- North America
- Europe
- Asia-Pacific
- South America
- Middle East & Africa
Competitive Landscape
Company Profiles: Detailed analysis of the major companies present in the Global Asthma Warning Sensors Market.Available Customization
The analyst offers customization according to your specific needs. The following customization options are available for the report:- Detailed analysis and profiling of additional market players (up to five).
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
The key players profiled in this Asthma Warning Sensors market report include:- Philips Healthcare
- ResMed Inc.
- Teva Pharmaceutical Industries Ltd.
- GlaxoSmithKline PLC
- Novartis AG
- AstraZeneca PLC
- Merck & Co., Inc.
- Propeller Health
- Adherium Limited
- Cohero Health, Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 185 |
| Published | January 2026 |
| Forecast Period | 2025 - 2031 |
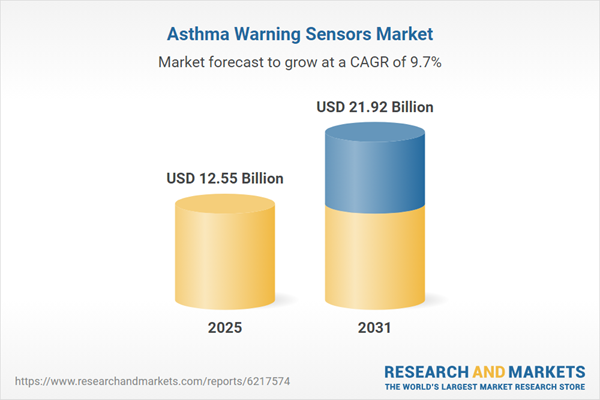
| Estimated Market Value ( USD | $ 12.55 Billion |
| Forecasted Market Value ( USD | $ 21.92 Billion |
| Compound Annual Growth Rate | 9.7% |
| Regions Covered | Global |
| No. of Companies Mentioned | 11 |