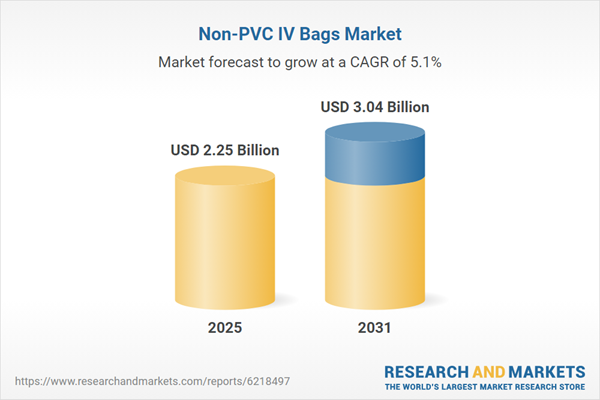
The non-PVC IV bags market is experiencing significant growth, driven by a fundamental shift toward safer and more sustainable materials in intravenous therapy. These bags, constructed from alternative polymers such as polyolefins (ethylene-vinyl acetate, polypropylene), polyurethane, and polyethersulfone, are designed to eliminate the potential health and environmental concerns associated with traditional polyvinyl chloride (PVC) containers. The market’s expansion is propelled by the convergence of rising clinical demand, stringent regulatory actions, and a broader industry movement toward eco-friendly healthcare solutions.
Core Market Drivers and Regulatory Catalysts
A primary growth driver is the escalating global burden of chronic diseases requiring intensive and long-term intravenous therapies. The rising prevalence of conditions such as cancer, chronic kidney disease (CKD), and other critical illnesses necessitates reliable, high-volume administration of fluids, chemotherapy, parenteral nutrition, and dialysis solutions. Non-PVC bags are increasingly specified for these applications due to their material inertness and compatibility with a wider range of drug formulations, making them a critical component in modern treatment protocols.
The most powerful and direct catalyst for market conversion is the global regulatory movement against di(2-ethylhexyl) phthalate (DEHP), a plasticizer commonly used to soften PVC. Regulatory bodies in major markets have issued advisories and restrictions on the use of DEHP-plasticized medical devices, particularly for vulnerable patient populations such as neonates, pregnant women, and critically ill individuals. These regulations stem from well-documented concerns over DEHP leaching, which can cause adverse metabolic and developmental effects. This regulatory pressure compels healthcare providers to seek safer alternatives, creating a mandatory shift in procurement and directly accelerating the adoption of non-PVC IV containers.
Concurrently, growing environmental and sustainability priorities within the healthcare sector are reinforcing this material transition. The environmental impact of PVC disposal, including challenges with recycling and potential release of toxins, has led healthcare systems to prioritize greener procurement policies. Non-PVC materials, which are often more readily recyclable and free of halogenated compounds, align with institutional sustainability goals and corporate social responsibility initiatives, adding an important non-clinical driver to the purchasing decision.
Clinical and Material Advantages
Beyond regulatory compliance, non-PVC IV bags offer distinct clinical and operational benefits. Their material composition generally provides superior compatibility with sensitive drug formulations, including certain lipids, taxanes, and other pharmaceuticals that can interact with or be absorbed by PVC. This reduces the risk of drug loss, potency reduction, and patient exposure to leached plasticizers. For healthcare facilities, this translates to enhanced patient safety, reduced medication waste, and greater flexibility in drug storage and administration.
Market Challenges and Restraints
The transition faces notable hurdles. A significant restraint is the higher unit cost of non-PVC bags compared to conventional PVC options. This cost differential can be a barrier for budget-constrained healthcare systems, particularly in price-sensitive markets, requiring a clear demonstration of long-term value through reduced complications and aligned sustainability benefits.
Furthermore, the entrenched, global manufacturing infrastructure for PVC medical devices presents a challenge to rapid market conversion. Supply chains for alternative polymers must achieve similar scale, reliability, and geographic penetration to meet global demand. Ensuring consistent material quality and navigating the complexities of validating new container-drug combinations also require ongoing investment from manufacturers.
Geographic Market Dynamics
North America is projected to maintain a leading market share, driven by a combination of stringent regulatory guidance, high awareness among healthcare providers, supportive reimbursement structures, and the presence of major market players. The region’s focus on patient safety, coupled with proactive environmental policies in healthcare, creates a highly receptive environment for non-PVC alternatives. Europe follows a similar trajectory, heavily influenced by comprehensive regulations like the EU Medical Device Regulation (MDR) and strong environmental mandates. The Asia-Pacific region represents a high-growth opportunity, fueled by expanding healthcare infrastructure, increasing treatment volumes, and a gradual rise in regulatory standards and environmental awareness, though price sensitivity remains a more pronounced factor.
Competitive Landscape and Strategic Direction
The market is characterized by the presence of large, established medical device companies with extensive portfolios in infusion therapy. Competition centers on material science innovation, drug compatibility data, supply chain reliability, and the ability to offer a comprehensive range of non-PVC solutions. Strategic activities include investment in advanced polymer manufacturing, securing regulatory approvals for specific high-value applications (e.g., oncology, neonatology), and forming partnerships with drug manufacturers to develop compatible packaging systems. Success depends on demonstrating clinical superiority, achieving cost efficiencies at scale, and providing robust educational support to facilitate the transition for healthcare providers.
In conclusion, the non-PVC IV bags market is transitioning from a niche alternative to a mainstream standard, driven by an irreversible regulatory and environmental mandate. Its growth is structurally linked to the expansion of intravenous therapy across chronic disease management and the healthcare industry's commitment to safer, more sustainable practices. For industry experts, the critical path forward involves continuing to advance material technologies to improve performance and reduce costs, generating comprehensive clinical data to support broader adoption, and navigating the complex global regulatory landscape to ensure seamless market access. The long-term trajectory points toward non-PVC materials becoming the dominant standard for IV containment, reflecting the evolving priorities of patient care and environmental stewardship.
Key Benefits of this Report:
- Insightful Analysis: Gain detailed market insights covering major as well as emerging geographical regions, focusing on customer segments, government policies and socio-economic factors, consumer preferences, industry verticals, and other sub-segments.
- Competitive Landscape: Understand the strategic maneuvers employed by key players globally to understand possible market penetration with the correct strategy.
- Market Drivers & Future Trends: Explore the dynamic factors and pivotal market trends and how they will shape future market developments.
- Actionable Recommendations: Utilize the insights to exercise strategic decisions to uncover new business streams and revenues in a dynamic environment.
- Caters to a Wide Audience: Beneficial and cost-effective for startups, research institutions, consultants, SMEs, and large enterprises.
What can this report be used for?
Industry and Market Insights, Opportunity Assessment, Product Demand Forecasting, Market Entry Strategy, Geographical Expansion, Capital Investment Decisions, Regulatory Framework & Implications, New Product Development, Competitive Intelligence.Report Coverage:
- Historical data from 2022 to 2024 & forecast data from 2025 to 2031
- Growth Opportunities, Challenges, Supply Chain Outlook, Regulatory Framework, and Trend Analysis
- Competitive Positioning, Strategies, and Market Share Analysis
- Revenue Growth and Forecast Assessment of segments and regions including countries
- Company Profiling (Strategies, Products, Financial Information, and Key Developments among others.
Non-PVC IV Bags Market Segmentation:
- By Type
- Single Chamber
- Multi-Chamber
- By Material
- Ethylene Vinyl Acetate (EVA)
- Polypropylene (PP)
- Copolyester Ether
- By Capacity
- Up to 50 ml
- 50 to 100 ml
- Greater than 100 ml
- By End-User
- Hospitals
- Specialty Clinics
- Others
- By Geography
- North America
- United States
- Canada
- Mexico
- South America
- Brazil
- Argentina
- Others
- Europe
- Germany
- France
- United Kingdom
- Spain
- Others
- Middle East and Africa
- Saudi Arabia
- UAE
- Others
- Asia-Pacific
- China
- India
- Japan
- South Korea
- Indonesia
- Thailand
- Others
- North America
Table of Contents
Companies Mentioned
The companies profiled in this Non-PVC IV Bags market report include:- B. Braun SE
- ICU Medical, Inc
- Fresenius Kabi AG
- Technoflex
- Silica Healthcare Private Limited
- Spirit Medical Limited
- Ningbo Siny Medical Technology Co.
- Rusoma Laboratories Private Limited
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 141 |
| Published | January 2026 |
| Forecast Period | 2025 - 2031 |
| Estimated Market Value ( USD | $ 2.25 Billion |
| Forecasted Market Value ( USD | $ 3.04 Billion |
| Compound Annual Growth Rate | 5.1% |
| Regions Covered | Global |
| No. of Companies Mentioned | 9 |