Radioligand therapy (RLT) utilizes targeted ligands to deliver therapeutic radionuclides to cancer cells, and is now clinically and regulatorily validated for the treatment of advanced prostate cancer expressing prostate-specific membrane antigen (PSMA-positive) and somatostatin receptor (SSTR)-expressing neuroendocrine tumors (NETs). Although the science dates back decades, commercial momentum has accelerated in recent years, with Novartis AG’s Pluvicto (Lu-177 PSMA) and Lutathera (Lu-177 SSTR) now anchoring today’s approved market. Radioligand therapy can produce durable benefits in selected patients, but it requires specialized infrastructure and careful management of treatment-related effects and radiation safety.
The field is rapidly evolving. Access to prostate-specific membrane antigen positron emission tomography (PSMA PET) and somatostatin receptor positron emission tomography (SSTR PET), the shift to earlier-line settings and combination regimens with standard care are expanding the eligible patient populations. At the same time, the supply of therapeutic isotopes and trained nuclear medicine personnel remains a significant constraint, especially outside leading centers. RLT in cancer treatment is expected to grow across North America, Europe and emerging markets through 2030, with the approved Lutetium-177 (Lu-177) products supporting near-term revenues and alpha-emitter and novel platform programs offering mid-term growth potential.
Report Scope
This report provides an in-depth analysis of the global market for radioligand therapeutics (RLT) in cancer treatment. It presents detailed market data for 2024 (base year), estimates for 2025 and forecasts with compound annual growth rates (CAGR) through 2030. The study evaluates current market dynamics, emerging trends and future growth potential driven by the adoption of prostate-specific membrane antigen (PSMA)- and somatostatin receptor (SSTR)-targeted therapies.The report assesses the competitive environment, including product-level analysis of approved radioligand therapies such as 177Lu-vipivotide tetraxetan (Pluvicto) and 177Lu-dotatate (Lutathera), as well as pipeline assets targeting PSMA, SSTR, gastrin‐releasing peptide receptor (GRPR) and other tumor-specific receptors. It also examines regulatory pathways and reimbursement frameworks.
Market segmentation encompasses product type, indication type, and end user, with additional assessments of drivers, restraints, opportunities, technological advances, and strategic activities such as collaborations, acquisitions, clinical trial investments, and expansion of isotope production capacity.
Segmental-level market data (by product type, indication type and end user) is limited to the North America region. This limitation highlights that the market for radioligand therapeutics in cancer treatment is centered in the U.S., where a single company’s products (Pluvicto and Lutathera from Novartis AG) account for the majority of global sales and where reliable, detailed data are accessible. In other regions, RLT is still in an early stage, with limited commercial adoption and insufficient transparent data to enable detailed segment-level analysis.
The report includes:
- 15 data tables and 57 additional tables
- Overview and an analysis of the global markets for radioligand therapeutics (RLT) in cancer treatment
- Analyses of the global market trends, with historic revenue (sales data) from 2022 to 2024, estimates for 2025 and projections of CAGRs through 2030
- Estimates of the current market size and revenue prospects for the global market, along with a corresponding market share analysis based on product type, disease indication, end user and region
- Facts and figures about market dynamics, opportunities and deterrents, technological advances, regulations, prospects and the impact of macroeconomic variables
- Highlights of promising new advancements in precision oncology, the emergence of innovative and effective theranostic agents, applications and potential
- Insights derived from Porter’s Five Forces model, as well as global supply chain analyses
Table of Contents
Companies Mentioned
- Abdera Therapeutics
- Ariceum Therapeutics
- Clarity Pharmaceuticals
- Convergent Therapeutics Inc.
- Curium
- Fusion Pharma
- Itm Isotope Technologies Munich SE
- Lilly Usa LLC.
- Novartis AG
- Orano Med
- Perspective Therapeutics
- Precirix
- Radiopharm Theranostics Ltd.
- Rayzebio Inc.
- Telix Pharmaceuticals Ltd.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 106 |
| Published | February 2026 |
| Forecast Period | 2025 - 2030 |
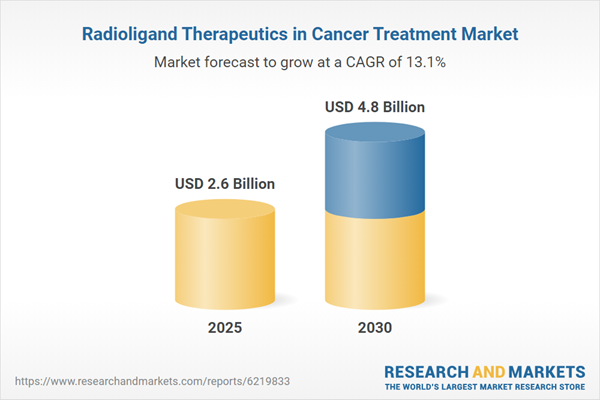
| Estimated Market Value ( USD | $ 2.6 Billion |
| Forecasted Market Value ( USD | $ 4.8 Billion |
| Compound Annual Growth Rate | 13.1% |
| Regions Covered | Global |
| No. of Companies Mentioned | 15 |