This new report, Biomarkers: Technologies and Global Markets, provides a comprehensive analysis of the biomarkers market in a global context, including market forecasts and sales through 2025. The report analyzes the market, segmenting it into various product offerings (i.e., instruments, consumables [reagents, kits and panels], services and software). Segmentation also provides analysis by popular technology type (genomics, proteomics and metabolomics, imaging and bioinformatics). This study surveys the biomarker market by therapeutic area (cancer, cardiovascular and metabolic diseases, infectious diseases, neurodegenerative diseases, autoimmune diseases and others. End-users include academic institutes, pharma and biotechnology companies, clinical research organizations, hospitals and diagnostics. Geographic regions include North America, Europe and Emerging markets. Emerging markets include countries like India, China, Korea, Taiwan, Africa, Australia, New Zealand, Canada, Latin America, etc.
This report features new product developments and patents that are boosting global growth in this market. This report provides comprehensive profiles of market players in the industry. The industry structure chapter focuses on changing market trends, market players and leading products. This chapter also covers mergers and acquisitions and other collaborations or partnerships that are expected to shape the industry.
Strengths, weaknesses, threats and opportunities are expected to play a role in the diagnostic biomarkers market. These are evaluated in detail.
The scope of the report excludes in vitro diagnostic products and regulatory aspects. Digital biomarkers are not covered in this report.
The report includes:
- 48 data tables and 25 additional tables
- An updated review and current landscape of the global biomarkers market
- Analyses of the global market trends, with data from 2018-2020, and projections of compound annual growth rates (CAGRs) through 2025
- Estimation of market size and market potential for global biomarkers market, and corresponding market share analysis by product type, technology type, therapeutic area, end-user and geographic region for each market segment
- Latest information on market opportunities and drivers, industry structure, regulatory frameworks, clinical trials and technological updates which are affecting the overall market growth
- Identification of the leading biotechnology companies poised to introduce products during the forecast period, their impact on the face of the competitive environment and research priorities
- Encompassing details of major types of biomarkers and their use in clinical trial assessment, drug discovery and development, and therapeutics
- Review of patents issued for biomarker technologies and deep dive of the patent data by year, technology type, application, company, assignee and applicant country
- Profile description of major market players, including Abbott Laboratories Ltd., Bayer AG, Bio-Rad Laboratories Inc., Canon Medical Corp., and Luminex Corp.
Table of Contents
Chapter 1 Introduction
- Study Goals and Objectives
- Reasons for Doing This Study
- Scope of Report
- Information Sources
- Methodology
- Geographic Breakdown
- Analyst's Credentials
- Custom Research
- Related Reports
Chapter 2 Summary and Highlights
- Key Highlights
Chapter 3 Market and Technology Background
- Definitions of Biomarkers
- Classification of Biomarkers
- Examples of Biomarkers
- Surrogate Biomarkers/Endpoints
- Types of Biomarkers
- Molecular Biomarkers
- Imaging Biomarkers
- Biomarker Discovery, Verification and Validation
- Biomarker Discovery and Development
- Biomarker Verification
- Biomarker Validation
- Clinical Implementation
- Biomarker Qualification
- Technologies Used in Biomarker Analysis
- Genomics
- Proteomics
- Metabolomics
- Imaging
- Bioinformatics
- Applications of Biomarkers
- Diagnostics, Therapeutics and Disease Monitoring
- Drug Discovery and Development
- Clinical Trials
- Personalized Medicine
Chapter 4 Market Breakdown by Product Type
- Market by Biomarker Product Type
- Market Overview
- Market Revenue
- Market Share
- Market for Biomarker Products by Region
- Market Shares
- Instruments
- Consumables
- Services
- Software
Chapter 5 Market Breakdown by Technology Type
- Biomarker Market by Technology Type
- Market Overview
- Market Revenue
- Market Share
- Genomics
- Proteomics
- Metabolomics
- Imaging
- Bioinformatics
Chapter 6 Market Breakdown by Therapeutic Area
- Market by Therapeutic Area
- Market Overview
- Market Revenue
- Market Share
- Cancer
- Cardiovascular and Metabolic Diseases
- Infectious Diseases
- Neurodegenerative Diseases
- Autoimmune Diseases
- Other Diseases
Chapter 7 Market Breakdown by End User
- Market by Biomarker End-User
- Market Overview
- Market Revenue
- Market Share
- Academic Institutes
- Pharma and Biotechnology Companies
- Clinical Research Organizations (CROs)
- Hospitals and Diagnostics
Chapter 8 Industry Structure
- Industry Trends
- Collaborations and Partnerships
- License Agreements
- Mergers and Acquisitions
- Leading Manufacturers/Suppliers of Biomarkers Technologies
- Genomics
- Proteomics
- Metabolomics
- Imaging
- Bioinformatics
Chapter 9 Clinical Trials
- Clinical Trials by Therapeutic Area
- Clinical Trials by Study Status
- Clinical Trials by Study Phase
- Clinical Trials of Studies including Children
- Clinical Trials by Country
- Clinical Trials by Sponsor
Chapter 10 Patent Analysis
- Patents on Biomarkers
- Case Studies
- Case Study: Association for Molecular Pathology v. Myriad Genetics Inc.
- Case Study: Roche Molecular Systems Inc. v. Cepheid
- Patent Analysis
- Patents by Year
- Patents by Type
- Patents by Application
- Patents by Company
- Patents, by Country
- Patents by Type of Assignee
Chapter 11 Analysis of Market Opportunities
- Strengths of the Biomarker Market
- Rising Incidence of Diseases
- Drug Development Costs and Failures
- Advances in Omics and Imaging Technologies
- Support from Regulatory Agencies
- Collaborations and Partnerships
- Challenges in the Biomarker Market
- Technological Challenges
- Regulatory Challenges
- Requirement of Skilled Labor
- High Development Cost of Biomarkers
- Reimbursement Challenges
- Opportunities in the Biomarker Market
- Emerging Markets
- Personalized Medicine
- Innovation in Technology
- Funding and Research Initiatives
- Threats to the Biomarker Market
- Competition
- Regulatory and Reimbursement Challenges
- COVID-19 Pandemic
Chapter 12 Company Profiles
- 10X Genomics
- Abbott Laboratories Ltd.
- Abcam Plc
- Agilent Technologies Inc.
- Bayer Ag
- Beckman Coulter Life Sciences
- Becton Dickinson & Co.
- Biolegend
- Bio-Rad Laboratories Inc.
- Bio-Techne Corp.
- Bruker Corp.
- Canon Medical Systems Corp.
- Cepheid
- Creative Proteomics
- Cubresa Inc.
- Cytiva
- Danaher Corp.
- Fujifilm Visual Sonics Inc.
- GE Healthcare
- F Hoffmann-La Roche Ag
- Illumina Inc.
- Integrated DNA Technologies (IDT)
- Jeol Ltd.
- Koninklijke Philips N.V.
- Lantheus Medical Imaging Inc.
- Leco Corp.
- Leica Biosystems
- Luminex Corp.
- Metabolon Inc.
- Millipore Sigma
- Molecular Devices
- Oxford Nanopore Technologies Ltd.
- Pacific Biosciences Inc.
- Perkinelmer Inc.
- Promega Corp.
- Qiagen N.V.
- Quanterix Corp.
- Sciex
- Siemens Healthineer Ag
- Shimadzu Corp.
- Thermo Fisher Scientific Inc.
- Waters Corp.
Chapter 13 Appendix: Abbreviations/Acronyms
List of Tables
Summary Table: Global Market for Biomarkers, by Technology, Through 2025
Table 1: Different Categories of Biomarkers
Table 2: Examples of Biomarkers
Table 3: Different Types of ELISAs
Table 4: Global Market for Biomarkers, by Product Type, Through 2025
Table 5: Global Market Shares of Biomarkers, by Product Type, 2019
Table 6: Global Market for Biomarkers, by Region, Through 2025
Table 7: Global Market Shares of Biomarkers, by Region, 2019
Table 8: Global Market for Biomarker Instruments, by Region, Through 2025
Table 9: Global Market for Biomarker Consumables, by Region, Through 2025
Table 10: Global Market for Biomarker Services, by Region, Through 2025
Table 11: Global Market for Biomarker Software, by Region, Through 2025
Table 12: Global Market for Biomarkers, by Technology Type, Through 2025
Table 13: Global Market Shares of Biomarkers, by Technology Type, 2019
Table 14: Global Market for Genomics Biomarkers, by Region, Through 2025
Table 15: Global Market for Proteomics Biomarkers, by Region, Through 2025
Table 16: Global Market for Metabolomics Biomarkers, by Region, Through 2025
Table 17: Global Market for Imaging Biomarkers, by Region, Through 2025
Table 18: Global Market for Bioinformatics in Biomarkers, by Region, Through 2025
Table 19: Global Market for Biomarkers, by Therapeutic Area, Through 2025
Table 20: Global Market Shares of Biomarkers, by Therapeutic Area, 2019
Table 21: Global Market for Cancer Biomarkers, by Region, Through 2025
Table 22: Global Market for Cardiovascular and Metabolic Disease Biomarkers, by Region, Through 2025
Table 23: Global and U.S. Prevalence, Common Infectious Diseases
Table 24: Global Market for Infectious Disease Biomarkers, by Region, Through 2025
Table 25: Global Market for Neurodegenerative Disease Biomarkers, by Region, Through 2025
Table 26: Global Market for Autoimmune Disease Biomarkers, by Region, Through 2025
Table 27: Biomarkers for Other Diseases
Table 28: Global Market for Biomarkers for Other Diseases, by Region, Through 2025
Table 29: Global Market for Biomarkers, by End User, Through 2025
Table 30: Global Market Shares of Biomarkers, by End User, 2019
Table 31: Global Market for Biomarkers in Academic Institutes, by Region, Through 2025
Table 32: Global Market for Biomarkers in Pharma and Biotechnology, by Region, Through 2025
Table 33: Global Market for Biomarkers in CROs, by Region, Through 2025
Table 34: Global Market for Biomarkers in Hospitals and Diagnostics, by Region, Through 2025
Table 35: Alliances in Global Biomarker Market, by Year, 2018-2020
Table 36: Biomarker-Focused Consortia, 2002-2017
Table 37: Collaborations/Partnerships in the Global Biomarker Market, 2018-January 2021
Table 38: Licensing Agreements in the Global Biomarker Market, 2018-January 2021
Table 39: Mergers and Acquisitions in the Global Biomarker Market, 2018-January 2021
Table 40: Leading Manufacturers/Suppliers of Genomic Technologies, 2019
Table 41: Global Market Shares of Leading Manufacturers/Suppliers of Genomic Technologies, 2019
Table 42: Leading Manufacturers/Suppliers of Proteomic Technologies, 2019
Table 43: Global Market Shares of Leading Manufacturers/Suppliers of Proteomics Technologies, 2019
Table 44: Leading Manufacturers/Suppliers, Metabolomic Technologies, 2019
Table 45: Global Market Shares of Leading Manufacturers/Suppliers of Metabolomic Technologies, 2019
Table 46: Leading Manufacturers/Suppliers, Imaging Technologies, 2019
Table 47: Global Market Shares of Leading Manufacturers/Suppliers of Imaging Technologies, 2019
Table 48: Leading Manufacturers/Suppliers of Bioinformatics Technologies, 2019
Table 49: Global Market Shares of Leading Manufacturers/Suppliers of Bioinformatics Technologies, 2019
Table 50: Clinical Trials in Biomarkers, by Therapeutic Area, 2018-2020
Table 51: Clinical Trials in Biomarkers, by Study Status, 2018-2020
Table 52: Clinical Trials in Biomarkers, by Phase of the Study, 2018-2020
Table 53: Clinical Trials in Biomarkers, Studies including Children, 2018-2020
Table 54: Clinical Trials in Biomarkers, by Country, 2018-2020
Table 55: Clinical Trials in Biomarkers, by Sponsor, 2018-2020
Table 56: Representative Patents in Biomarkers, 2018-December 15, 2020
Table 57: Patents Issued for Biomarkers, by Year, 2018-December 15, 2020
Table 58: Patents Issued for Biomarkers, by Type, 2018-December 15, 2020
Table 59: Patents Issued for Biomarkers, by Application, 2018-December 15, 2020
Table 60: Patents Issued for Biomarkers, by Company, 2018-December 15, 2020
Table 61: Patents Issued for Biomarkers, by Country, 2018-December 15, 2020
Table 62: Patents Issued for Biomarkers, by Type of Assignee, 2018-December 15, 2020
Table 63: Biomarkers with Letters of Support from the EMA, 2018-2020
Table 64: Companion Diagnostics and Approved Drugs, 2017-2020
Table 65: FoundationOne CDx Approved Indications
Table 66: Abbott Laboratories: Biomarker Clinical Trials
Table 67: Bayer: Biomarker Clinical Trials
Table 68: Bio-Rad Laboratories: Biomarker Clinical Trials
Table 69: GE Healthcare: Biomarker Clinical Trials
Table 70: F Hoffmann-La Roche: Biomarker Clinical Trials
Table 71: Millipore-Sigma: Biomarker Clinical Trials
Table 72: Abbreviations in Biomarker Technologies and Global Markets Report
List of Figures
Summary Figure: Global Market for Biomarkers, by Technology, 2018-2025
Figure 1: Different Categories of Biomarkers
Figure 2: Molecular and Imaging Biomarkers
Figure 3: Biomarker Discovery, Verification and Validation
Figure 4: Biomarker Qualification Process
Figure 5: Global Market for Biomarkers, by Product Type, 2018-2025
Figure 6: Global Market Shares of Biomarkers, by Product Type, 2019
Figure 7: Global Market for Biomarkers, by Region, 2018-2025
Figure 8: Global Market Shares of Biomarkers, by Region, 2019
Figure 9: Global Market for Biomarker Instruments, by Region, 2018-2025
Figure 10: Global Market for Biomarker Consumables, by Region, 2018-2025
Figure 11: Global Market for Biomarker Services, by Region, 2018-2025
Figure 12: Global Market for Biomarker Software, by Region, 2018-2025
Figure 13: Global Market for Biomarkers, by Technology Type, 2018-2025
Figure 14: Global Market Shares of Biomarkers, by Technology Type, 2019
Figure 15: Global Market for Genomics Biomarkers, by Region, 2018-2025
Figure 16: Global Market for Proteomics Biomarkers, by Region, 2018-2025
Figure 17: Global Market for Metabolomics Biomarkers, by Region, 2018-2025
Figure 18: Global Market for Imaging Biomarkers, by Region, 2018-2025
Figure 19: Global Market for Bioinformatics in Biomarkers, by Region, 2018-2025
Figure 20: Global Market for Biomarkers, by Therapeutic Area, 2018-2025
Figure 21: Global Market Shares of Biomarkers, by Therapeutic Area, 2019
Figure 22: Global Market for Cancer Biomarkers, by Region, 2018-2025
Figure 23: Global Market for Cardiovascular and Metabolic Disease Biomarkers, by Region, 2018-2025
Figure 24: Global Market for Infectious Disease Biomarkers, by Region, 2018-2025
Figure 25: Global Market for Neurodegenerative Disease Biomarkers, by Region, 2018-2025
Figure 26: Global Market for Autoimmune Disease Biomarkers, by Region, 2018-2025
Figure 27: Global Market for Biomarkers for Other Diseases, by Region, 2018-2025
Figure 28: Global Market for Biomarkers, by End User, 2018-2025
Figure 29: Global Market Shares of Biomarkers, by End User, 2019
Figure 30: Global Market for Biomarkers in Academic Institutes, by Region, 2018-2025
Figure 31: Global Market for Biomarkers in Pharma and Biotechnology, by Region, 2018-2025
Figure 32: Global Market for Biomarkers in CROs, by Region, 2018-2025
Figure 33: Global Market for Biomarkers in Hospitals and Diagnostics, by Region, 2018-2025
Figure 34: Global Market Shares of Leading Manufacturers/Suppliers of Genomic Technologies, 2019
Figure 35: Global Market Shares of Leading Manufacturers/Suppliers of Proteomics Technologies, 2019
Figure 36: Global Market Shares of Leading Manufacturers/Suppliers of Metabolomic Technologies, 2019
Figure 37: Global Market Shares of Leading Manufacturers/Suppliers of Imaging Technologies, 2019
Figure 38: Global Market Shares of Leading Manufacturers/Suppliers of Bioinformatic Technologies, 2019
Figure 39: Clinical Trials in Biomarkers, by Therapeutic Area, 2018-2020
Figure 40: Clinical Trials in Biomarkers, by Study Status, 2018-2020
Figure 41: Clinical Trials in Biomarkers, by Phase of the Study, 2018-2020
Figure 42: Clinical Trials in Biomarkers, Studies including Children, 2018-2020
Figure 43: Patents Issued for Biomarkers, by Year, 2018-December 15, 2020
Figure 44: Patents Issued for Biomarkers, by Type, 2018-December 15, 2020
Figure 45: Patents Issued for Biomarkers, by Application, 2018-December 15, 2020
Figure 46: Patents Issued for Biomarkers, by Country, 2018-December 15, 2020
Figure 47: Patents Issued for Biomarkers, by Type of Assignee, 2018-December 15, 2020
Figure 48: Global Market for Biomarker SWOT Analysis
Companies Mentioned
- 10X Genomics
- Abbott Laboratories Ltd.
- Abcam Plc
- Agilent Technologies Inc.
- Bayer Ag
- Beckman Coulter Life Sciences
- Becton Dickinson & Co.
- Bio-Rad Laboratories Inc.
- Bio-Techne Corp.
- Biolegend
- Bruker Corp.
- Canon Medical Systems Corp.
- Cepheid
- Creative Proteomics
- Cubresa Inc.
- Cytiva
- Danaher Corp.
- F Hoffmann-La Roche Ag
- Fujifilm Visual Sonics Inc.
- GE Healthcare
- Illumina Inc.
- Integrated DNA Technologies (IDT)
- Jeol Ltd.
- Koninklijke Philips N.V.
- Lantheus Medical Imaging Inc.
- Leco Corp.
- Leica Biosystems
- Luminex Corp.
- Metabolon Inc.
- Millipore Sigma
- Molecular Devices
- Oxford Nanopore Technologies Ltd.
- Pacific Biosciences Inc.
- Perkinelmer Inc.
- Promega Corp.
- Qiagen N.V.
- Quanterix Corp.
- Sciex
- Shimadzu Corp.
- Siemens Healthineer Ag
- Thermo Fisher Scientific Inc.
- Waters Corp.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 216 |
| Published | March 2021 |
| Forecast Period | 2020 - 2025 |
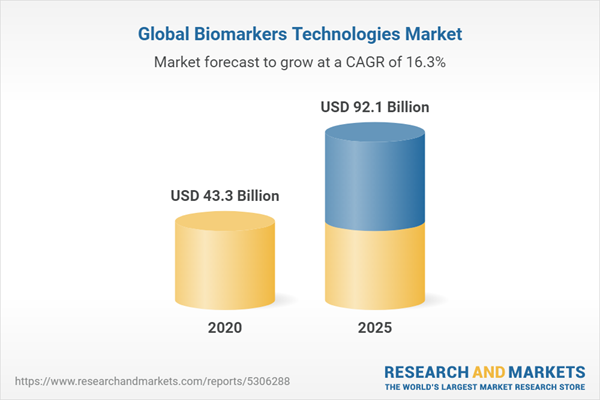
| Estimated Market Value ( USD | $ 43.3 Billion |
| Forecasted Market Value ( USD | $ 92.1 Billion |
| Compound Annual Growth Rate | 16.3% |
| Regions Covered | Global |
| No. of Companies Mentioned | 42 |