The COVID-19 pandemic positively impacted the rapid diagnostics market in terms of revenue; however, the pandemic negatively impacted the sales of routine testing kits used for non-COVID-19 diseases and lockdowns. For instance, in April 2021, the Federal Government of Germany made it mandatory for employers in Gendatory for employers in Germany to offer free COVID-19 self-testing kits to employees not working from home once a week. Further, in March 2021, The German Federal Ministry of Health approved the sale of COVID-19 rapid antigen tests in supermarket chains, drug stores, pharmacies, and e-commerce websites. Additionally, in March 2021, Becker's Healthcare published that the United States government launched a national testing strategy to control the spread of COVID-19. Through this testing strategy, the Government opened four COVID-19 testing hubs nationwide with an investment of USD 650 million.
Moreover, the launches of several COVID-19 diagnostics kits and government investments in COVID-19 research further boosted the market growth during the pandemic. However, in the post-pandemic period, the number of COVID-19 infections decreased, and the research, diagnosis, and treatment of other infectious and chronic diseases resumed, leading to the market's growth. Thus, COVID-19 boosted the growth of the market initially. However, the market is growing normally with the decrease in the COVID-19 infection and the resumption of rapid diagnosis of other infectious and chronic diseases.
Furthermore, the rapid diagnostic kits market is expected to grow during the forecast period with the increasing government initiatives for diagnosing infectious diseases, rising awareness regarding early diagnosis among people, and shifts in technological and commercial environments.
In the past few years, infection tracking and surveillance programs have increased to track the infection rate of certain pathogens. These programs utilize rapid diagnostic assays to detect and confirm infections. Thus, with such programs, the demand for rapid diagnostic assays is expected to increase, likely to drive market growth. The Early and timely identification of infectious diseases propels disease surveillance programs and is important for vector control measures. Due to their easy accessibility and quick turnaround time for results, rapid test kits are increasingly being utilized to track recent emerging infections. For instance, in February 2021, the Centers for Disease Control and Prevention (CDC) reported 7,860 cases of Tuberculosis in the United States (a rate of 2.4 per 100,000 persons).
Further, in May 2021, according to the data published by the TB Facts Org 2021, over 10.6 million were estimated to be affected with Tuberculosis (TB) in the year 2021, which showed an increase of 4.5% compared to the previous year. Furthermore, while microscopy and culture remain essential for tuberculosis laboratory diagnosis, the spectrum of rapid diagnostic test kits, particularly the Nucleic Acid Amplification Test (NAAT), has greatly expanded. For instance, The World Health Organization (WHO) in 2021 recommended the use of molecular NAATs for Tuberculosis detection instead of smear microscopy since they detect Tuberculosis more correctly, especially in patients with paucibacillary illness and persons living with Human immunovirus (HIV). They are becoming more widely available for detecting and identifying mycobacterium tuberculosis complex in clinical specimens and for diagnosing multi-drug resistant strains. Further, in March 2022, Brain Chemistry Labs, a non-profit research institute, and Arlington Scientific, a Utah-based medical test kit manufacturer, announced the development of an easy-to-use rapid test kit. This kit will detect the presence of β-methylamino-L-Alanine (BMAA), a toxin in cyanobacterial blooms, which causes a fatal paralytic disease called Amyotrophic Lateral Sclerosis (ALS). Thus, with such developments, the penetration of rapid diagnostic assays is expected to increase further, driving the market's growth in the coming years.
Additionally, the increasing government initiatives for diagnosing and researching infectious diseases are boosting the growth of the studied market. For instance, in September 2021, the Government of New Zealand invested over USD 22.47 million (NZD 36 million) in a new Infectious Diseases Research Platform to support Aotearoa New Zealand's Covid-19 response and preparedness for future pandemics. Further, in September 2022, the Government of Australia invested over USD 179.02 million (AUD 266 million) for three years into the seventh replenishment of the global fund to control acquired immunodeficiency syndrome (AIDS), malaria, and Tuberculosis.
Therefore, owing to the factors above, including the increasing prevalence of infectious diseases, increasing government investment in research, and the key developments by the market players, the studied market is anticipated to witness growth over the analysis period. However, the lack of awareness regarding newer rapid diagnostic tests and failure to eliminate the need for microscopy diagnosis will likely impede market growth.
Rapid Diagnostic Kits Market Trends
The Hospitals and Clinics Segment is Expected to Witness Significant Growth Over the Forecast Period
The hospital and clinics segment is anticipated to offer significant growth during the forecast period, owing to the advanced medical infrastructure of the hospitals and clinics for the rapid diagnosis of infectious and chronic diseases.Several foreign hospital groups from developed countries and emerging economies are collaborating with diagnostic companies to offer patients effective and extensive diagnostic services. For instance, in June 2022, Kingston Hospital NHS Foundation Trust collaborated with the Royal Marsden Partners to launch the Rapid Diagnostic Cancer Clinic (RDCC) in the United Kingdom. The newly launched facility would offer rapid diagnosis services for timely cancer detection in the United Kingdom. Further, in May 2021, Aster DM Healthcare signed a strategic partnership with Roche Diagnostics. Under the partnership, both companies signed a Memorandum of Understanding (MoU) to expand their diagnostic services in the United Arab Emirates, Saudi Arabia, Qatar, and Oman. Through the partnership, Aster DM Healthcare'sHealthcare's hospitals, clinics, and laboratories across the region would access the advanced diagnostic technology of Roche Diagnostics. The partnership would allow Aster Hospitals and Roche to continue fulfilling their commitment to preventing, diagnosing, and treating various disease areas, including cardiovascular health, women's health, and infectious diseases. Also, in December 2022, the Department of Health launched two rapid diagnosis centers at Whiteabbey Hospital and South Tyrone Hospital of North Ireland to fast-track cancer diagnosis. With such developments, the market penetration of rapid diagnosis is expected to increase in hospitals and clinics, which is likely to drive the segment'ssegment's growth in the coming years.
Therefore, the hospital and clinical testing segment is expected to grow during the forecast period, with factors including recent developments.
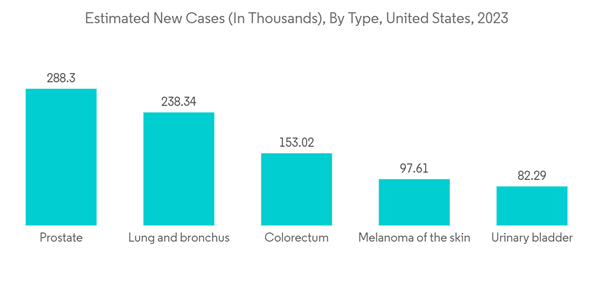
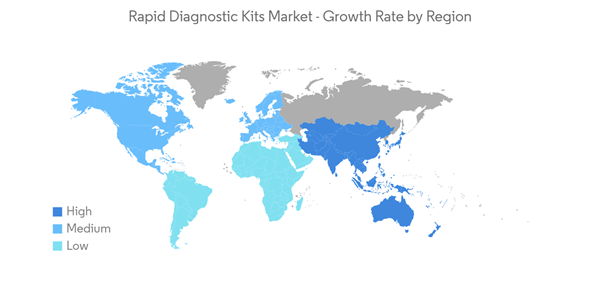
North America is Expected to Witness Significant Growth Over the Forecast Period
North America is expected to witness significant growth over the forecast period, owing to the recent development by the market players, the high prevalence of chronic and infectious diseases, and high investments in the research and diagnosis of infectious diseases.The region has a significant burden of chronic and infectious diseases, which is expected to create more demand for rapid diagnostic tests in the coming years. For instance, according to the data published by the American Cancer Society in 2023, over 18 million Americans were reported to have cancer in 2022. The same source stated that over 1.9 million new cancer cases are estimated to be diagnosed in the United States in 2023. Further, per the data published by the Canadian Cancer Society in November 2022, over 233,900 new cancer cases were estimated in 2021. Also, the burden of infectious diseases is high in the region, which is also expected to drive the growth of the regional rapid diagnostic kits market. For instance, as per the data published by Statistics Canada in March 2023, over 1,904 active tuberculosis cases were reported in Canada in 2021. Further, as per the data published by the World Bank in 2023, the incidence of tuberculosis per 1 million individuals in Mexico was reported to be 25 in 2021. Thus, such a high burden of chronic and infectious diseases is likely to increase the demand for rapid testing kits for the rapid and timely detection of diseases, which is expected to bolster regional market growth.
Furthermore, the rising government investments in researching and diagnosing infectious and chronic diseases in this region will also likely boost market growth. For instance, in August 2022, the United States Agency for International Development (USAID) launched its project, Supporting, Mobilizing, and Accelerating Research for Tuberculosis Elimination (SMART4TB). The project is a five-year initiative under which USAID would invest up to USD 200 million to identify more effective methods and tools for studying, treating, and preventing tuberculosis (TB) in USAID's 24 priority countries for TB programming. Further, in September 2022, the Government of Canada invested over USD 0.75 billion (CAD 1.21 billion) in the Global Fund to curb the acquired immunodeficiency syndrome (AIDS) is a chronic, potentially life-threatening condition caused by the human immunodeficiency virus (HIV), TB and Malaria.
Additionally, the key developments by the market players in rapid diagnostic kits in the region are expected to boost the regional market's growth. For instance, in September 2021, Becton, Dickinson, and Company signed a collaboration with Scanwell Health to develop an at-home rapid test for SARS-CoV-2 using a BD antigen test and the Scanwell Health mobile app. Further, in December 2021, Roche received the Emergency Use Authorization (EUA) from the USFDA for its COVID-19 at-home test, which offers rapid, accurate, and reliable detection of the SARS-CoV-2 and all known variants of concern, including Omicron in 20 minutes. Hence, such developments in the market are anticipated to increase the penetration of rapid diagnostic kits in the region, which is expected to boost the growth of the regional market.
Therefore, the studied market is anticipated to grow in the North America Region due to the factors above, including the high burden of chronic diseases, key developments, and government investments in diagnosing and researching infectious diseases.
Rapid Diagnostic Kits Industry Overview
The rapid diagnostic kits market is competitive in nature due to the presence of several companies operating globally as well as regionally. The key players operating in the market include ACON Laboratories Inc., Abbott Laboratories, Alfa Scientific Designs Inc, Artron Laboratories Inc., and F. Hoffmann-La Roche Ltd.Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
This product will be delivered within 2 business days.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Abbott Laboratories
- ACON Laboratories Inc.
- Alfa Scientific Designs Inc.
- Artron Laboratories Inc.
- Becton, Dickinson and Company
- BioMerieux
- BTNX Inc.
- Creative Diagnostics
- Danaher Corporation
- F. Hoffmann-La Roche Ltd.
- Hologic Inc.
- Siemens Healthcare GmbH