COVID-19 had a significant impact on the growth of the market. The global carrier screening market faced limitations in development due to the sudden imposition of the worldwide lockdowns, which reduced the patient influx to genetic counselors. According to the study published in Prenatal Diagnosis in July 2021, the COVID-19 pandemic influenced pregnant women's decisions about prenatal genetic testing. Patients' access to and use of prenatal genetic tests impacted the pandemic. However, genetic testing for COVID-19 diagnosis increased during the pandemic. However, the relaxation of the restrictions during the post-pandemic period contributed to the market's growth. For instance, a study published in the European Journal of Human Genetics in November 2022 indicated that between 2020 and 2022, willingness to take a genetic test climbed considerably. Thus the market studied is expected to grow over the forecast period.
The significant factors for the growth of the carrier screening market include the increasing emphasis on early disease detection and prevention and the rising application of screening tests in genetic disorders, which is expected to experience during the forecast period. For instance, the Centers for Disease Control and Prevention (CDC) data updated in May 2022 reported that sickle cell disease affects millions of people throughout the world and is particularly common among those ancestors who came from sub-Saharan Africa, Spanish-speaking regions, such as South America, the Caribbean, and Central America, as well as Saudi Arabia, India, and Mediterranean countries.
Additionally, the rising demand for personalized medicine and various activities taken by market players are expected to boost the market growth. For instance, in May 2021, AdventHealth expanded its genomics program with the launch of a data-driven precision medicine initiative that would structure and integrate longitudinal patient data with molecular testing data, creating a dataset and disease network models to help the health system better predict the development of diseases and responses to treatments. Moreover, increased product launches by the market players are expected to support the market expansion. For instance, in June 2021, Grail launched the Galleri blood test, a groundbreaking multi-cancer screening diagnostic. The test was meant to screen people who may already have an elevated risk for cancer, such as adults over the age of 50 years.
Thus, all the factors above, such as the rising prevalence of genetic diseases and the increasing adoption of precision medicine, are expected to boost the market over the forecast period. However, the social and ethical implications and the high costs and reimbursement issues of carrier testing are restraining the market's growth.
Carrier Screening Market Trends
Molecular Screening Test Segment is Expected to Register Significant Growth during the Forecast Period
A molecular screening test identifies DNA mutations, which are variations in the genetic code that lead to decreased production of enzymes. It focuses on the mutations seen in one ethnic group. It involves a stepwise testing process for common alleles and, if required, extensive gene analysis. Sequencing is a method of molecular screening accomplished by reading across the DNA code of a specific gene to know if there are any known mutations. If the test results are negative, it reduces the chances that the individual is a carrier. However, it does not eliminate the possibility of having a carrier gene since the mutation might still need to be discovered through the current technology.According to the Minority of HIV/AIDS Fund (MHAF), United States Department of Health and Human Services Statistics, updated in June 2021, estimated that approximately 37.6 million people were living with HIV worldwide in 2020, of whom 35.9 million were adults and 1.7 million were children (under the age of 15). According to the same source, 1.5 million people were estimated to have contracted HIV globally in 2020. The market is expanding due to the rising prevalence of HIV and the resulting demand for carrier screening. The increasing need for screening tests results from the high prevalence of viral illnesses.
Furthermore, globally, there is an increasing cancer incidence, driving the market growth. For instance, according to Cancer Facts and Figures 2022, published in January 2022 by the American Cancer Society, an estimated 1.9 million new cancer cases would be diagnosed in 2022, among which prostate cancer is estimated to be 186,670, followed by 169,870 cases of lung cancer, and 144,490 cases of female breast cancer. Therefore, the global incidence of cancer and modern healthcare facilities have been major drivers of the segment's growth.
There is an expansion of molecular testing, as it has the potential to increase testing accuracy through technical benefits for many targeted disorders that may not be suggested for biochemical testing. Thus, the abovementioned factors are expected to drive market growth over the forecast period.
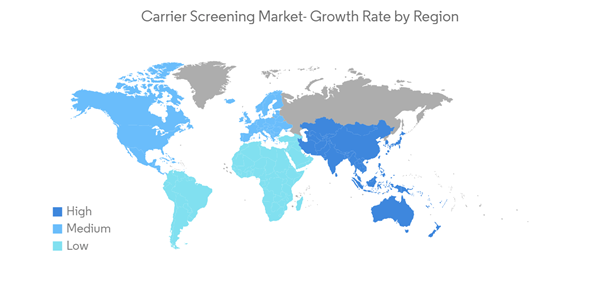
North America is Expected to Hold a Significant Share in the Market and Expected to do Same in the Forecast Period
North America holds a major share of the carrier screening market and is expected to show a similar trend over the forecast period without significant fluctuations.According to statistics published by the Government of Canada and released in November 2021, about 229,200 Canadians were diagnosed with cancer in 2021. Prostate cancer was expected to remain the most diagnosed cancer, accounting for 46% of all cancer diagnoses in 2021. According to the same source, breast cancer affected one out of every eight women at some point. Thus, the growing prevalence of cancer is expected to increase demand for carrier screening, thereby boosting market growth.
The increasing product approvals by the US Food and Drug Administration (FDA) and subsequent launches, along with the high concentration of key players involved in research activities for the innovation of novel genetic testing products, are anticipated to drive market growth in North America. For instance, in January 2022, Mitera announced the release of its Peaches & Me and 23Pears at-home reproductive genetic testing kits in all 50 states. Peaches & Me is the first non-invasive prenatal test (NIPT) that patients can order online and have administered at home. As early as ten weeks of pregnancy, it can screen for disorders like Down syndrome and determine the baby's gender.
Thus, considering the above factors, such as the rising prevalence of cancer and product launches, is expected to fuel market growth in the North American region over the forecast period.
Carrier Screening Industry Overview
The Carrier Screening Market is fragmented and competitive and consists of several major players. In terms of market share, a few major players dominate the market. The presence of major market players, such as Abbott, F. Hoffmann-La Roche AG, Danaher Corporation (Cepheid), Illumina Inc., and Thermo Fisher Scientific Inc, is increasing the overall competitive rivalry of the market.Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
This product will be delivered within 2 business days.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- 23Andme Inc.
- Abbott Laboratories
- F. Hoffmann-La Roche AG
- Cepheid (Danaher Corporation)
- Illumina Inc.
- Luminex Corporation
- Sequenom Inc. (Laboratory Corporation of America Holdings)
- Myriad Genetics
- Autogenomics Inc.
- Thermo Fisher Scientific Inc.