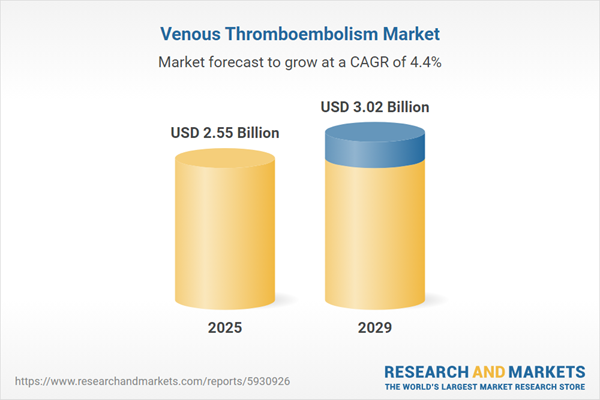
The venous thromboembolism market size has grown steadily in recent years. It will grow from $2.44 billion in 2024 to $2.55 billion in 2025 at a compound annual growth rate (CAGR) of 4.4%. The growth in the historic period can be attributed to aging population, advancements in anticoagulants, reimbursement policies, increasing investments.
The venous thromboembolism market size is expected to see steady growth in the next few years. It will grow to $3.02 billion in 2029 at a compound annual growth rate (CAGR) of 4.4%. The growth in the forecast period can be attributed to global healthcare access, genetic risk assessment, telemedicine and remote monitoring, patient education and support. Major trends in the forecast period include extended thromboprophylaxis, novel oral anticoagulants, personalized treatment plans, advanced diagnostic imaging.
The expansion of healthcare infrastructure is expected to drive the growth of the venous thromboembolism (VTE) market. Healthcare infrastructure refers to the systems, institutions, policies, and resources that enable the delivery of medical care, including prevention, treatment, and rehabilitation services. The growing healthcare industry supports the implementation of VTE prevention protocols and the development of related treatments. For example, in May 2023, the American Health Care Association reported an increase in the number of hospitals in the U.S., rising from 6,093 in 2022 to 6,129 in 2023. Similarly, the Office for National Statistics in the UK reported a 5.6% increase in healthcare expenditure, which reached £292 billion in 2023. Additionally, long-term health and social care expenditures rose by 2.8% in real terms in 2022. As healthcare systems expand, they will increasingly focus on VTE prevention, thus boosting the market for related products and services.
The growth of the venous thromboembolism market is further bolstered by the escalating number of orthopedic procedures. Orthopedic procedures encompass various surgical interventions used to address conditions affecting the spine, joints, and skeletal deformities. Procedures like total knee arthroplasty (TKA), total hip arthroplasty (THA), and hip fracture surgery (HFS) are associated with a heightened risk of VTE, leading to an increased adoption of compression devices for DVT prophylaxis. Compression devices serve to prevent the formation of blood clots in the deep veins of the legs, mitigating the risk of venous thromboembolism (VTE). For instance, research published by the National Library of Medicine in February 2023 indicates that THA and TKA procedures are projected to rise significantly, with 1,222,988 and 719,364 procedures, respectively, anticipated by 2040. Moreover, TKAs are estimated to reach 2,917,959 by 2060, while THAs are projected to total 1,982,099 by that time. Consequently, the mounting number of orthopedic procedures is a significant driver of the venous thromboembolism market's growth.
Leading companies within the venous thromboembolism market are spearheading the development of innovative anticoagulant drugs and solutions to maintain their market positions. Advances in anticoagulants hold the potential to yield more effective drugs capable of preventing or treating venous thromboembolism. For example, in September 2022, Anthos Therapeutics, a US-based clinical-stage biotherapeutics company, secured regulatory approval for its abelacimab anticoagulant medication from the Food and Drug Administration (FDA). This fully human monoclonal antibody is indicated for stroke and systemic embolism prevention in atrial fibrillation patients. The newly developed abelacimab, a once-monthly dual-acting antibody, is designed to provide hemostasis-sparing anticoagulation by inhibiting Factor XI.
In August 2022, Boston Scientific Corporation, a US-based leader in surface modification technologies, acquired Obsidio, Inc., a US-based company specializing in biodegradable embolic materials for treating venous conditions, for an undisclosed amount. This acquisition strengthens Boston Scientific’s portfolio of innovative therapies, expanding its capabilities in minimally invasive procedures aimed at patients with venous conditions. The acquisition is expected to enhance the company’s offerings in the growing field of venous health, particularly with Obsidio’s cutting-edge materials designed to improve treatment outcomes for patients suffering from conditions like venous thromboembolism (VTE).
Major companies operating in the venous thromboembolism market are Pfizer Inc., Johnson and Johnson Limited, Merck and Co. Inc., AbbVie Inc., Bayer AG, Novartis AG, Sanofi-Aventis LLC., Bristol-Myers Squibb Company, GlaxoSmithKline plc., Eli Lilly and Co Ltd., Boehringer Ingelheim International GmbH, Teva Pharmaceutical Industries Ltd., Daiichi Sankyo Company Limited, Sun Pharmaceutical Industries Limited, Otsuka Pharmaceutical Co. Ltd., Menarini Group, Ipsen S.A., Dr. Reddy's Laboratories Ltd., Apotex Inc., Hikma Pharmaceuticals PLC, Cipla Inc., Endo International PLC, Aspen Pharmacare Holdings Limited, Zydus Lifesciences Ltd., LEO Pharma A/S, Lupin Limited, Torrent Pharmaceuticals Ltd., Almirall S.A., Glenmark Pharmaceuticals Ltd., Portola Pharmaceuticals Inc.
North America was the largest region in the venous thromboembolism market in 2024. The regions covered in venous thromboembolism report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East and Africa. The countries covered in the venous thromboembolism market report are Australia, Brazil, China, France, Germany, India, Indonesia, Japan, Russia, South Korea, UK, USA, Canada, Italy, Spain.
The venous thromboembolism market consists of revenues earned by entities by providing services such as surgical assistance and thrombolytic therapy. The market value includes the value of related goods sold by the service provider or included within the service offering. The venous thromboembolism market also includes the sales of heparin, apixaban, dabigatran, rivaroxaban, edoxaban and warfarin. Values in this market are ‘factory gate’ values, that is, the value of goods sold by the manufacturers or creators of the goods, whether to other entities (including downstream manufacturers, wholesalers, distributors and retailers) or directly to end customers. The value of goods in this market includes related services sold by the creators of the goods.
Venous thromboembolism (VTE) is a medical condition marked by the development of blood clots within the veins, carrying the potential for serious or life-threatening complications. Common indicators of VTE encompass symptoms like leg pain and swelling, rapid breathing, chest discomfort, and more. Diverse treatment methods, including anticoagulant medications, medical devices, and thrombolytic therapy, are employed in managing VTE.
The primary categories of venous thromboembolism consist of deep vein thrombosis, pulmonary embolism, and other related conditions. Deep vein thrombosis (DVT) represents a medical condition characterized by the development of blood clots within the deep veins of the body. Treatment options encompass the use of anticoagulant medications, mechanical devices, thrombolytic therapy, and various other interventions. These treatments cater to diverse end-users, including hospitals, homecare settings, specialty centers, and other relevant healthcare facilities.
The venous thromboembolism market research report is one of a series of new reports that provides venous thromboembolism market statistics, including venous thromboembolism industry global market size, regional shares, competitors with a venous thromboembolism market share, detailed venous thromboembolism market segments, market trends and opportunities and any further data you may need to thrive in the venous thromboembolism industry. This venous thromboembolism market research report delivers a complete perspective of everything you need, with an in-depth analysis of the current and future scenario of the industry.
The market value is defined as the revenues that enterprises gain from the sale of goods and/or services within the specified market and geography through sales, grants, or donations in terms of the currency (in USD unless otherwise specified).
The revenues for a specified geography are consumption values that are revenues generated by organizations in the specified geography within the market, irrespective of where they are produced. It does not include revenues from resales along the supply chain, either further along the supply chain or as part of other products.
This product will be delivered within 3-5 business days.
Table of Contents
Executive Summary
Venous Thromboembolism Global Market Report 2025 provides strategists, marketers and senior management with the critical information they need to assess the market.This report focuses on venous thromboembolism market which is experiencing strong growth. The report gives a guide to the trends which will be shaping the market over the next ten years and beyond.
Reasons to Purchase:
- Gain a truly global perspective with the most comprehensive report available on this market covering 15 geographies.
- Assess the impact of key macro factors such as conflict, pandemic and recovery, inflation and interest rate environment and the 2nd Trump presidency.
- Create regional and country strategies on the basis of local data and analysis.
- Identify growth segments for investment.
- Outperform competitors using forecast data and the drivers and trends shaping the market.
- Understand customers based on the latest market shares.
- Benchmark performance against key competitors.
- Suitable for supporting your internal and external presentations with reliable high quality data and analysis
- Report will be updated with the latest data and delivered to you along with an Excel data sheet for easy data extraction and analysis.
- All data from the report will also be delivered in an excel dashboard format.
Description
Where is the largest and fastest growing market for venous thromboembolism? How does the market relate to the overall economy, demography and other similar markets? What forces will shape the market going forward? The venous thromboembolism market global report answers all these questions and many more.The report covers market characteristics, size and growth, segmentation, regional and country breakdowns, competitive landscape, market shares, trends and strategies for this market. It traces the market’s historic and forecast market growth by geography.
- The market characteristics section of the report defines and explains the market.
- The market size section gives the market size ($b) covering both the historic growth of the market, and forecasting its development.
- The forecasts are made after considering the major factors currently impacting the market. These include:
- The forecasts are made after considering the major factors currently impacting the market. These include the Russia-Ukraine war, rising inflation, higher interest rates, and the legacy of the COVID-19 pandemic.
- Market segmentations break down the market into sub markets.
- The regional and country breakdowns section gives an analysis of the market in each geography and the size of the market by geography and compares their historic and forecast growth. It covers the growth trajectory of COVID-19 for all regions, key developed countries and major emerging markets.
- The competitive landscape chapter gives a description of the competitive nature of the market, market shares, and a description of the leading companies. Key financial deals which have shaped the market in recent years are identified.
- The trends and strategies section analyses the shape of the market as it emerges from the crisis and suggests how companies can grow as the market recovers.
Scope
Markets Covered:
1) By Type: Deep Vein Thrombosis; Pulmonary Embolism; Other Types2) By Treatment: Anti-Clotting Medications; Mechanical Devices; Thrombolytic Therapy; Other Treatment
3) By End Users: Hospitals; Homecare; Specialty Centers; Other End Users
Subsegments:
1) By Deep Vein Thrombosis (DVT): Proximal DVT; Distal DVT; Asymptomatic DVT2) By Pulmonary Embolism (PE): Acute PE; Chronic PE; Submassive PE
3) By Other Types: Thrombophlebitis; Superficial Vein Thrombosis; Post-Thrombotic Syndrome
Key Companies Mentioned: Pfizer Inc.; Johnson and Johnson Limited; Merck and Co. Inc.; AbbVie Inc.; Bayer AG
Countries: Australia; Brazil; China; France; Germany; India; Indonesia; Japan; Russia; South Korea; UK; USA; Canada; Italy; Spain
Regions: Asia-Pacific; Western Europe; Eastern Europe; North America; South America; Middle East; Africa
Time Series: Five years historic and ten years forecast.
Data: Ratios of market size and growth to related markets, GDP proportions, expenditure per capita.
Data Segmentation: Country and regional historic and forecast data, market share of competitors, market segments.
Sourcing and Referencing: Data and analysis throughout the report is sourced using end notes.
Delivery Format: PDF, Word and Excel Data Dashboard.
Companies Mentioned
Some of the major companies featured in this Venous Thromboembolism market report include:- Pfizer Inc.
- Johnson and Johnson Limited
- Merck and Co. Inc.
- AbbVie Inc.
- Bayer AG
- Novartis AG
- Sanofi-Aventis LLC.
- Bristol-Myers Squibb Company
- GlaxoSmithKline plc.
- Eli Lilly and Co Ltd.
- Boehringer Ingelheim International GmbH
- Teva Pharmaceutical Industries Ltd.
- Daiichi Sankyo Company Limited
- Sun Pharmaceutical Industries Limited
- Otsuka Pharmaceutical Co. Ltd.
- Menarini Group
- Ipsen S.A.
- Dr. Reddy's Laboratories Ltd.
- Apotex Inc.
- Hikma Pharmaceuticals PLC
- Cipla Inc.
- Endo International plc
- Aspen Pharmacare Holdings Limited
- Zydus Lifesciences Ltd.
- LEO Pharma A/S
- Lupin Limited
- Torrent Pharmaceuticals Ltd.
- Almirall S.A.
- Glenmark Pharmaceuticals Ltd.
- Portola Pharmaceuticals Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 200 |
| Published | March 2025 |
| Forecast Period | 2025 - 2029 |
| Estimated Market Value ( USD | $ 2.55 Billion |
| Forecasted Market Value ( USD | $ 3.02 Billion |
| Compound Annual Growth Rate | 4.4% |
| Regions Covered | Global |
| No. of Companies Mentioned | 31 |