Global Venous Thromboembolism Treatment Market - Key Trends & Drivers Summarized
What Is Causing A Surge In Therapeutic Innovation Around VTE Management?
Venous thromboembolism (VTE), comprising deep vein thrombosis (DVT) and pulmonary embolism (PE), continues to present a substantial clinical challenge due to its prevalence, recurrence risk, and potentially fatal outcomes. The condition affects nearly 10 million people annually worldwide, with mortality often resulting from undiagnosed or undertreated PE. Over the past decade, therapeutic protocols have undergone significant change, propelled by an evolution in pharmacological agents, diagnostic accuracy, and interventional strategies. The shift away from warfarin toward direct oral anticoagulants (DOACs) has been particularly influential, offering patients fixed dosing, fewer dietary restrictions, and reduced need for monitoring.Regulatory approvals of DOACs such as rivaroxaban, apixaban, and edoxaban have redefined first-line treatment pathways. These agents are now widely recommended in guidelines by ACCP, ESC, and NICE for both acute and extended VTE management. Simultaneously, there has been a growing emphasis on individualized treatment regimens based on bleeding risk, renal function, and cancer co-morbidities. Novel agents targeting Factor XIa and tissue factor pathway inhibitors are in late-stage clinical trials, offering the potential to reduce thrombotic risk with minimal bleeding liability. This next wave of anticoagulants aims to bridge the gap between efficacy and safety in high-risk populations.
How Are Interventional and Supportive Approaches Reshaping the VTE Treatment Landscape?
Beyond anticoagulant pharmacotherapy, the treatment of VTE is increasingly incorporating endovascular techniques, particularly for severe or recurrent cases. Catheter-directed thrombolysis (CDT), pharmacomechanical thrombectomy, and balloon angioplasty are now considered in select patients with iliofemoral DVT or life-threatening PE. These interventions aim to restore venous patency, reduce post-thrombotic syndrome, and prevent chronic thromboembolic pulmonary hypertension (CTEPH). Integration of intravascular ultrasound (IVUS), clot fragmentation technologies, and real-time perfusion imaging is improving procedural precision, expanding eligibility among patients previously deemed unsuitable for invasive treatment.In parallel, mechanical prophylactic tools such as intermittent pneumatic compression (IPC) devices, graduated compression stockings, and inferior vena cava (IVC) filters remain essential adjuncts in surgical wards, ICU settings, and trauma centers. Advanced analytics and predictive algorithms are being embedded into electronic health records (EHRs) to identify patients at highest VTE risk and trigger preventative measures. Wearable biosensors and AI-driven platforms are also emerging, helping monitor adherence and coagulation markers in outpatient settings, thereby facilitating remote VTE management and relapse prevention.
Where Is Demand for VTE Therapies Rising, and Which Patient Populations Are Driving It?
The demand for VTE treatment is increasing globally, but especially in aging societies with high surgical and cancer burdens. The United States and Europe lead in terms of treatment volumes, supported by structured VTE prevention programs in hospitals, widespread availability of DOACs, and a growing number of interventional radiologists trained in clot removal techniques. In Asia-Pacific, however, the market is rapidly catching up, driven by improving diagnostic infrastructure and expanded health insurance coverage for anticoagulants and diagnostic imaging modalities like duplex ultrasound and CT angiography.High-risk groups fueling the VTE treatment market include post-operative orthopedic patients, cancer patients undergoing chemotherapy, pregnant women, and individuals with genetic clotting disorders such as Factor V Leiden. Notably, oncology patients represent a major focus area as cancer-associated thrombosis has emerged as a significant contributor to morbidity. Pharma companies are tailoring studies to evaluate VTE outcomes in this cohort, while hospitals are implementing risk-adjusted prophylaxis protocols in oncology wards. The pandemic-induced rise in thrombotic complications among hospitalized COVID-19 patients has also intensified focus on early detection and intervention in inflammatory and critical care settings.
What Forces Are Powering Growth in the Global VTE Treatment Market?
The growth in the venous thromboembolism treatment market is driven by several factors, including the shift toward DOAC-based management, rising surgical interventions, and expanding awareness among both physicians and patients. Healthcare systems are investing in early detection protocols to reduce VTE-related readmissions and fatalities. National policies-such as NHS England’s Commissioning for Quality and Innovation (CQUIN) framework and U.S. CMS mandates-are reinforcing systematic VTE risk assessments and preventive action plans in clinical settings.Pharmaceutical pipeline activity is robust, with multiple late-stage anticoagulant candidates showing promise for reducing bleeding complications. Market players are also investing in co-packaging solutions and fixed-dose combinations to improve adherence. On the device side, thrombectomy platforms and compression systems are benefiting from increased procedural volume in tertiary care and ambulatory surgery centers. Public health education and DTC campaigns are further amplifying treatment-seeking behavior. Collectively, these dynamics point to a sustained and diversified expansion of the global VTE treatment market across drugs, devices, and digital platforms.
Scope of the Report
The report analyzes the Venous Thromboembolism Treatment market, presented in terms of market value (USD). The analysis covers the key segments and geographic regions outlined below:- Segments: Product (Non-Segmented Pneumatic Compression Pumps, Segmented Pneumatic Compression Pumps without Gradient, Segmented Pneumatic Compression Pumps with Calibrated Gradient, Upper Pneumatic Compression Sleeves, Lower Pneumatic Compression Sleeves, Permanent Inferior Vena Cava Filters, Other Products); Indication (Deep Venous Thrombosis Indication, Pulmonary Embolism Indication); End-User (Hospitals End-User, Catheterization Laboratories End-User, Ambulatory Surgery Centers End-User).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Non-Segmented Pneumatic Compression Pumps segment, which is expected to reach US$826.2 Million by 2030 with a CAGR of a 5.0%. The Segmented Pneumatic Compression Pumps without Gradient segment is also set to grow at 2.8% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $610.6 Million in 2024, and China, forecasted to grow at an impressive 7.2% CAGR to reach $570.3 Million by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Venous Thromboembolism Treatment Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Venous Thromboembolism Treatment Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Venous Thromboembolism Treatment Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Abbott Laboratories, Alexion (AstraZeneca Rare Disease), AngioDynamics, Aspen Pharmacare, AstraZeneca and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 42 companies featured in this Venous Thromboembolism Treatment market report include:
- Abbott Laboratories
- Alexion (AstraZeneca Rare Disease)
- AngioDynamics
- Aspen Pharmacare
- AstraZeneca
- Bayer AG
- Bayer AG (vascular devices division)
- Bristol-Myers Squibb
- Cipla Inc.
- Daiichi Sankyo
- Dr. Reddy’s Laboratories
- Eli Lilly and Co.
- GlaxoSmithKline
- Hikma Pharmaceuticals
- Inari Medical
- Johnson & Johnson
- Merck & Co.
- Mylan (now part of Viatris)
- Novartis AG
- Pfizer Inc.
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025 (E), competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Abbott Laboratories
- Alexion (AstraZeneca Rare Disease)
- AngioDynamics
- Aspen Pharmacare
- AstraZeneca
- Bayer AG
- Bayer AG (vascular devices division)
- Bristol-Myers Squibb
- Cipla Inc.
- Daiichi Sankyo
- Dr. Reddy’s Laboratories
- Eli Lilly and Co.
- GlaxoSmithKline
- Hikma Pharmaceuticals
- Inari Medical
- Johnson & Johnson
- Merck & Co.
- Mylan (now part of Viatris)
- Novartis AG
- Pfizer Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 384 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
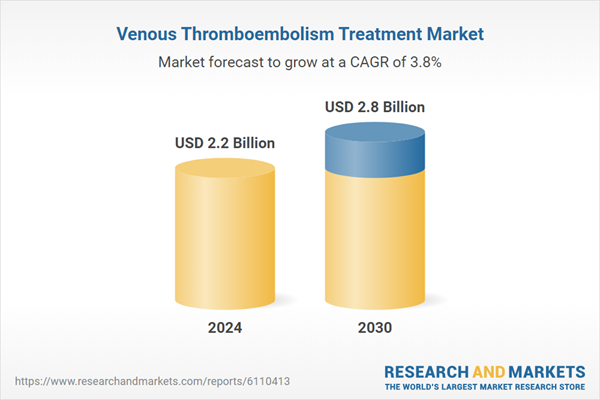
| Estimated Market Value ( USD | $ 2.2 Billion |
| Forecasted Market Value ( USD | $ 2.8 Billion |
| Compound Annual Growth Rate | 3.8% |
| Regions Covered | Global |