Speak directly to the analyst to clarify any post sales queries you may have.
A succinct strategic introduction to the craniomaxillofacial device environment driven by clinical demand, regulatory tightening, and technological integration
The craniomaxillofacial device landscape is at an inflection point driven by converging clinical demands, technological maturation, and evolving regulatory expectations. Clinicians increasingly require solutions that deliver predictable surgical outcomes while minimizing operative time and long-term complications, and this demand sits alongside institutional priorities to optimize cost of care and procedure throughput. As a result, device developers are refining portfolios to balance ease of use with advanced biological performance, integrating digital planning, patient-specific design, and materials science innovations.
In parallel, regulatory bodies have sharpened scrutiny around device traceability, post-market surveillance, and the clinical evidence that supports novel materials and manufacturing methods. This regulatory evolution compels manufacturers to invest earlier in clinical validation and quality systems, accelerating the convergence between product development and clinical research pathways. Consequently, the market environment rewards organizations that can demonstrate robust safety profiles, reproducible manufacturing processes, and clear clinical value propositions.
Financial and strategic stakeholders are also recalibrating investment priorities toward platforms that reduce procedural variability and enable personalized care pathways. Therefore, success now requires multidisciplinary alignment across R&D, clinical affairs, quality, and commercial teams, with an emphasis on scalable manufacturing and interoperable digital ecosystems. Taken together, these dynamics establish the operating context for manufacturers, providers, and investors focused on craniomaxillofacial interventions.
How personalization, advanced materials, and data-driven clinical workflows are redefining product development and adoption in craniomaxillofacial care
The landscape for craniomaxillofacial devices is undergoing transformative shifts that are reshaping product lifecycles, sourcing strategies, and clinical workflows. Advances in additive manufacturing and digital planning tools are enabling a move from standardized, one-size-fits-all implants toward patient-specific solutions that improve anatomical fit and reduce intraoperative adjustments. This evolution is accompanied by broader adoption of image-guided surgery systems and integrated surgical planning platforms that streamline the preoperative-to-intraoperative continuum.
Simultaneously, material science innovations are expanding the palette of implantable options. Bioresorbable polymers and ceramic composites are being refined to deliver predictable degradation profiles and osteoconductive properties, while established metals continue to be optimized for surface treatments and design geometries that enhance osseointegration. These material advances are tightly coupled to manufacturing capabilities; therefore, companies that harmonize material selection with validated production processes gain competitive advantage.
Healthcare providers are also reconfiguring care models, emphasizing ambulatory procedures and shorter hospital stays when clinically appropriate. This shift exerts pressure on device developers to prioritize implant systems that reduce operative complexity and support fast recovery. Additionally, heightened expectations for long-term patient outcomes are driving more rigorous post-market data collection and real-world evidence programs, which in turn inform iterative product improvements and regulatory submissions. Cumulatively, these shifts are catalyzing a new era of device differentiation grounded in personalization, materials performance, and evidence-driven adoption.
Assessment of tariff-driven procurement and manufacturing responses that influence device sourcing, localized production, and clinical continuity across care settings
The introduction of tariffs and trade policy shifts impacting medical device components and finished goods has amplified the importance of resilient supply chains for craniomaxillofacial manufacturers and healthcare providers. Increased import duties on select categories can raise landed costs for components such as precision-machined titanium implants, polymer-based fixation systems, and specialized instrumentation, thereby influencing procurement strategies at hospitals, dental clinics, and ambulatory surgical centers. In response, procurement teams are reassessing supplier portfolios, exploring nearshoring opportunities, and negotiating longer-term contracts to stabilize access to critical products.
Manufacturers are evaluating the cumulative impacts on production economics by considering alternative sourcing for raw materials, regionalizing manufacturing footprints, and consolidating component suppliers to capture scale efficiencies. These operational adjustments often necessitate capital investment and revalidation of manufacturing processes, which can extend development timelines but ultimately reduce vulnerability to external tariffs. Moreover, device developers with flexible manufacturing platforms-especially those leveraging localized additive manufacturing or modular machining centers-can adapt more rapidly to shifts in tariff regimes and minimize disruptions to clinical supply.
Clinics and hospitals face the dual challenge of maintaining clinical continuity while managing procurement budgets under tighter cost pressures. Clinical teams and purchasing departments are collaborating more closely to identify clinically equivalent alternatives and to prioritize devices with demonstrated value in reducing reoperation and complication rates. Regulatory and reimbursement considerations further shape these decisions, making transparent total cost of care analyses and robust clinical evidence key determinants in product selection as tariff-related cost dynamics continue to play out.
Comprehensive segmentation insights revealing where product categories, clinical applications, manufacturing technologies, and material science meet real-world care settings
A granular segmentation framework reveals where innovation, clinical needs, and commercial opportunity intersect across product categories, applications, technologies, materials, and end users. Based on Product Type, market is studied across Bone Plates, Bone Screws, Mesh, and Surgical Instruments, and each category demands distinct design priorities related to load-bearing performance, fixation mechanics, and intraoperative ergonomics. Based on Application, market is studied across Craniofacial Trauma, Dental Surgery, Orthognathic Surgery, and Reconstructive Surgery, which vary in patient acuity, implant complexity, and multidisciplinary care pathways.
Based on Technology, market is studied across 3D Printing, Biofabrication, Cad/Cam, and Conventional Shaping. The 3D Printing is further studied across Fused Deposition Modeling, Powder Bed Fusion, and Stereolithography. The Cad/Cam is further studied across Laser Sintering and Milling, and these technological distinctions influence design tolerances, surface finish options, and qualification strategies. Based on Material, market is studied across Bioresorbable Polymers, Ceramics, Peek, and Titanium. The Bioresorbable Polymers is further studied across Pga, Pla, and Plga. The Ceramics is further studied across Alumina, Hydroxyapatite, and Zirconia, and material selection drives biodegradation profiles, radiopacity, and long-term mechanical integrity.
Based on End User, market is studied across Ambulatory Surgical Centers, Dental Clinics, and Hospitals, each of which operates under different procurement cycles, procedural volumes, and clinical staffing models. Interpreting segmentation through these lenses highlights that innovation opportunities often arise at the intersection of technology and material selection for specific clinical applications, and that commercialization strategies must be tailored to the operational realities of each end-user setting.
Regional dynamics and regulatory realities that influence adoption patterns, supply chain localization, and commercial strategies across global healthcare markets
Regional dynamics shape regulatory approaches, supply chain architectures, and clinical adoption patterns across the Americas, Europe, Middle East & Africa, and Asia-Pacific. In the Americas, advanced clinical infrastructure and consolidated purchasing by large hospital systems drive demand for implants and instruments that demonstrate efficiency gains and clear clinical benefits. Regulatory pathways emphasize strong clinical evidence and post-market surveillance, encouraging manufacturers to invest in outcome studies and real-world evidence programs that support adoption by major clinical centers.
Europe, Middle East & Africa presents heterogeneous regulatory landscapes and varied reimbursement environments, which necessitate region-specific market entry strategies and adaptable pricing frameworks. Manufacturers targeting this region often prioritize modular product offerings and flexible commercial models to address divergent hospital procurement practices and reimbursement criteria. The regulatory emphasis on safety and traceability also promotes increased investment in serialized tracking and digital patient records.
Asia-Pacific is characterized by rapid adoption of digital surgical planning and a growing ecosystem of domestic manufacturers and contract producers. Cost sensitivity in some markets coexists with strong demand for technologically advanced, patient-specific solutions in urban tertiary care centers. Consequently, organizations operating in Asia-Pacific must balance competitive pricing with localized clinical support, training initiatives, and streamlined supply chains to capture clinical momentum while navigating diverse regulatory requirements across the region.
Competitive landscape analysis highlighting incumbent strengths, emerging specialists, and partnership models that shape clinical adoption and market positioning
Competitive dynamics in the craniomaxillofacial device space reflect a spectrum of incumbents and emerging specialists focusing on distinct competitive advantages. Established medical device manufacturers continue to leverage integrated portfolios, global distribution networks, and longstanding clinical relationships to support broad adoption across surgical disciplines. These organizations typically emphasize validated manufacturing processes, regulatory compliance, and comprehensive clinical support programs that reduce adoption friction and support hospital purchasing decisions.
At the same time, specialized innovators and startups are advancing differentiated offerings in additive manufacturing, patient-specific implant design, and bioactive surface technologies. These entrants often accelerate clinical acceptance through targeted collaborations with key opinion leaders and by demonstrating improved procedural workflows in specific applications such as complex reconstructive surgeries or dental implantology. Contract manufacturers and regional producers also play a pivotal role by enabling cost-efficient production and localized supply continuity, particularly where tariff pressures and logistics constraints drive nearshoring.
Strategic partnerships between device developers, digital planning software providers, and healthcare delivery systems are becoming more common, as they provide a route to integrated solutions that encompass preoperative planning, implant fabrication, and postoperative outcome tracking. For commercial leaders, sustaining competitive position requires not only product differentiation but also investments in service models, surgeon training, and evidence generation that together create defensible value propositions across care settings.
Actionable strategic recommendations that focus on clinical evidence, supply chain resilience, regulatory readiness, and integrated commercial offerings to drive adoption
Industry leaders should prioritize actions that accelerate clinical adoption while mitigating operational and regulatory risk. First, align R&D roadmaps with validated clinical needs by engaging multidisciplinary clinician advisors early and investing in targeted evidence generation that demonstrates improvements in operative efficiency and patient outcomes. Bridging preclinical innovation with pragmatic clinical trials and registries helps translate laboratory promise into trusted clinical performance.
Second, diversify and regionalize supply chains to reduce exposure to tariffs and logistics disruptions; this includes qualifying alternate suppliers, exploring nearshoring options, and implementing manufacturing platforms that can be retooled rapidly for different materials and design families. Concurrently, optimize quality systems and regulatory strategies to expedite approvals across jurisdictions, and invest in scalable validation protocols for additive manufacturing and other advanced production technologies.
Third, develop integrated commercial propositions that combine device performance with digital planning services, clinician training, and outcome monitoring. Such bundled solutions lower barriers to adoption by simplifying the clinical pathway and offering quantifiable value to institutional buyers. Finally, pursue strategic partnerships with software vendors, contract manufacturers, and clinical networks to accelerate market access and expand service capabilities without disproportionate capital outlay. Collectively, these steps will strengthen resilience and create differentiated pathways to adoption across diverse care environments.
Rigorous mixed-methods research design combining primary clinician insights, secondary evidence synthesis, and iterative validation for decision-ready intelligence
The research methodology combines structured primary research, rigorous secondary analysis, and iterative validation to ensure findings are evidence-based and actionable. Primary research comprises in-depth interviews with clinicians, procurement leaders, and device development experts, capturing first-hand perspectives on clinical workflows, unmet needs, and adoption barriers. These qualitative inputs are complemented by targeted expert consultations that probe technical feasibility, material behaviour, and manufacturing constraints for novel device concepts.
Secondary analysis synthesizes peer-reviewed literature, regulatory guidance, standards from relevant authorities, patent filings, and publicly available clinical studies to contextualize primary inputs and identify trends in materials, technologies, and clinical outcomes. Data triangulation is applied across sources to reconcile discrepancies and to strengthen the validity of conclusions. The methodology places a premium on traceable evidence chains and reproducible assumptions, ensuring that recommendations are grounded in demonstrable clinical and technical factors.
Quality control measures include cross-validation of interview transcripts, independent expert review of technology assessments, and iterative scenario testing for supply chain and regulatory variables. Segmentation logic is applied consistently across product types, clinical applications, technologies, materials, and end-user settings to facilitate comparative analysis and to support decision-relevant insights for stakeholders.
Concluding strategic synthesis that underscores personalization, materials performance, and operational resilience as pillars of sustained clinical and commercial success
In conclusion, the craniomaxillofacial device environment is being reshaped by convergent forces: personalization enabled by digital design and additive manufacturing, material innovations that tailor biological interactions, and regulatory expectations that demand clear clinical evidence and traceable manufacturing controls. These drivers create meaningful opportunities for organizations that can integrate clinical needs with robust production practices and evidence-generation strategies. Market leaders will be those who align technological capability with reproducible clinical benefit and operational resilience.
Supply chain and policy developments-such as tariff adjustments and regional manufacturing dynamics-underscore the importance of flexible production footprints and strong procurement partnerships. Meanwhile, end users from ambulatory surgical centers to tertiary hospitals will prioritize devices and service models that demonstrably reduce procedural complexity and improve patient recovery trajectories. To capture the emerging clinical opportunities, stakeholders must adopt multidisciplinary approaches that combine product innovation, clinician engagement, and targeted commercial models.
Ultimately, success in this sector depends on translating material and manufacturing advances into clinically validated solutions that are supported by outcome data, regulatory compliance, and adaptable supply chains. Organizations that execute on these dimensions will be best positioned to deliver sustainable clinical and commercial impact across global care settings.
Additional Product Information:
- Purchase of this report includes 1 year online access with quarterly updates.
- This report can be updated on request. Please contact our Customer Experience team using the Ask a Question widget on our website.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
17. China Craniomaxillofacial Devices Market
Companies Mentioned
The key companies profiled in this Craniomaxillofacial Devices market report include:- 3D Systems, Inc.
- B. Braun Melsungen AG
- GPC Medical Ltd.
- Inion Oy
- Johnson & Johnson Services, Inc.
- Karl Leibinger Medizintechnik GmbH & Co. KG
- KLS Martin Group
- Medtronic plc
- OsteoMed, LLC
- Stryker Corporation
- Xilloc Medical B. V.
- Zimmer Biomet Holdings, Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 190 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
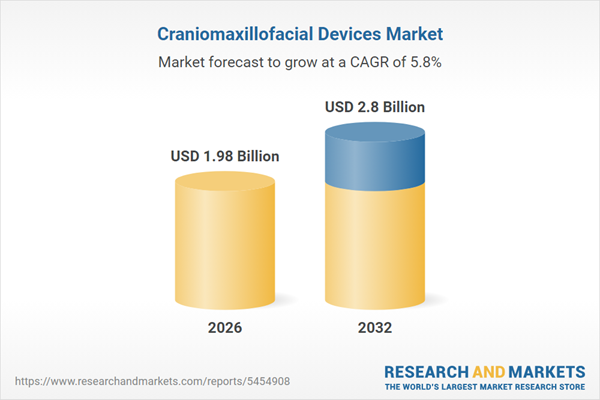
| Estimated Market Value ( USD | $ 1.98 Billion |
| Forecasted Market Value ( USD | $ 2.8 Billion |
| Compound Annual Growth Rate | 5.8% |
| Regions Covered | Global |
| No. of Companies Mentioned | 13 |