List of Tables
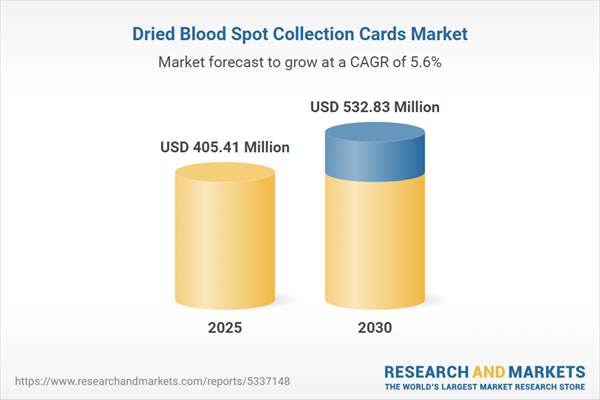
TABLE 1. GLOBAL DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, 2018-2030 (USD MILLION)
TABLE 2. GLOBAL DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY PRODUCT TYPE, 2018-2030 (USD MILLION)
TABLE 3. GLOBAL DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY FILTER PAPER CARDS, BY REGION, 2018-2030 (USD MILLION)
TABLE 4. GLOBAL DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY FILTER PAPER CARDS, BY GROUP, 2018-2030 (USD MILLION)
TABLE 5. GLOBAL DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY FILTER PAPER CARDS, BY COUNTRY, 2018-2030 (USD MILLION)
TABLE 6. GLOBAL DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY POLYMER COATED CARDS, BY REGION, 2018-2030 (USD MILLION)
TABLE 7. GLOBAL DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY POLYMER COATED CARDS, BY GROUP, 2018-2030 (USD MILLION)
TABLE 8. GLOBAL DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY POLYMER COATED CARDS, BY COUNTRY, 2018-2030 (USD MILLION)
TABLE 9. GLOBAL DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY TECHNOLOGY, 2018-2030 (USD MILLION)
TABLE 10. GLOBAL DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY ADVANCED DBS CARDS, BY REGION, 2018-2030 (USD MILLION)
TABLE 11. GLOBAL DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY ADVANCED DBS CARDS, BY GROUP, 2018-2030 (USD MILLION)
TABLE 12. GLOBAL DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY ADVANCED DBS CARDS, BY COUNTRY, 2018-2030 (USD MILLION)
TABLE 13. GLOBAL DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY STANDARD DBS CARDS, BY REGION, 2018-2030 (USD MILLION)
TABLE 14. GLOBAL DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY STANDARD DBS CARDS, BY GROUP, 2018-2030 (USD MILLION)
TABLE 15. GLOBAL DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY STANDARD DBS CARDS, BY COUNTRY, 2018-2030 (USD MILLION)
TABLE 16. GLOBAL DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY SAMPLE TYPE, 2018-2030 (USD MILLION)
TABLE 17. GLOBAL DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY PLASMA SPOTS, BY REGION, 2018-2030 (USD MILLION)
TABLE 18. GLOBAL DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY PLASMA SPOTS, BY GROUP, 2018-2030 (USD MILLION)
TABLE 19. GLOBAL DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY PLASMA SPOTS, BY COUNTRY, 2018-2030 (USD MILLION)
TABLE 20. GLOBAL DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY SERUM SPOTS, BY REGION, 2018-2030 (USD MILLION)
TABLE 21. GLOBAL DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY SERUM SPOTS, BY GROUP, 2018-2030 (USD MILLION)
TABLE 22. GLOBAL DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY SERUM SPOTS, BY COUNTRY, 2018-2030 (USD MILLION)
TABLE 23. GLOBAL DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY WHOLE BLOOD SPOTS, BY REGION, 2018-2030 (USD MILLION)
TABLE 24. GLOBAL DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY WHOLE BLOOD SPOTS, BY GROUP, 2018-2030 (USD MILLION)
TABLE 25. GLOBAL DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY WHOLE BLOOD SPOTS, BY COUNTRY, 2018-2030 (USD MILLION)
TABLE 26. GLOBAL DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY APPLICATION, 2018-2030 (USD MILLION)
TABLE 27. GLOBAL DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY CLINICAL DIAGNOSTICS, BY REGION, 2018-2030 (USD MILLION)
TABLE 28. GLOBAL DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY CLINICAL DIAGNOSTICS, BY GROUP, 2018-2030 (USD MILLION)
TABLE 29. GLOBAL DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY CLINICAL DIAGNOSTICS, BY COUNTRY, 2018-2030 (USD MILLION)
TABLE 30. GLOBAL DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY DISEASE SURVEILLANCE, BY REGION, 2018-2030 (USD MILLION)
TABLE 31. GLOBAL DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY DISEASE SURVEILLANCE, BY GROUP, 2018-2030 (USD MILLION)
TABLE 32. GLOBAL DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY DISEASE SURVEILLANCE, BY COUNTRY, 2018-2030 (USD MILLION)
TABLE 33. GLOBAL DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY FORENSIC TOXICOLOGY, BY REGION, 2018-2030 (USD MILLION)
TABLE 34. GLOBAL DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY FORENSIC TOXICOLOGY, BY GROUP, 2018-2030 (USD MILLION)
TABLE 35. GLOBAL DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY FORENSIC TOXICOLOGY, BY COUNTRY, 2018-2030 (USD MILLION)
TABLE 36. GLOBAL DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY NEWBORN SCREENING, BY REGION, 2018-2030 (USD MILLION)
TABLE 37. GLOBAL DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY NEWBORN SCREENING, BY GROUP, 2018-2030 (USD MILLION)
TABLE 38. GLOBAL DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY NEWBORN SCREENING, BY COUNTRY, 2018-2030 (USD MILLION)
TABLE 39. GLOBAL DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY NEWBORN SCREENING, 2018-2030 (USD MILLION)
TABLE 40. GLOBAL DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY CONGENITAL HYPOTHYROIDISM, BY REGION, 2018-2030 (USD MILLION)
TABLE 41. GLOBAL DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY CONGENITAL HYPOTHYROIDISM, BY GROUP, 2018-2030 (USD MILLION)
TABLE 42. GLOBAL DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY CONGENITAL HYPOTHYROIDISM, BY COUNTRY, 2018-2030 (USD MILLION)
TABLE 43. GLOBAL DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY CYSTIC FIBROSIS SCREENING, BY REGION, 2018-2030 (USD MILLION)
TABLE 44. GLOBAL DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY CYSTIC FIBROSIS SCREENING, BY GROUP, 2018-2030 (USD MILLION)
TABLE 45. GLOBAL DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY CYSTIC FIBROSIS SCREENING, BY COUNTRY, 2018-2030 (USD MILLION)
TABLE 46. GLOBAL DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY PHENYLKETONURIA, BY REGION, 2018-2030 (USD MILLION)
TABLE 47. GLOBAL DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY PHENYLKETONURIA, BY GROUP, 2018-2030 (USD MILLION)
TABLE 48. GLOBAL DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY PHENYLKETONURIA, BY COUNTRY, 2018-2030 (USD MILLION)
TABLE 49. GLOBAL DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY THERAPEUTIC DRUG MONITORING, BY REGION, 2018-2030 (USD MILLION)
TABLE 50. GLOBAL DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY THERAPEUTIC DRUG MONITORING, BY GROUP, 2018-2030 (USD MILLION)
TABLE 51. GLOBAL DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY THERAPEUTIC DRUG MONITORING, BY COUNTRY, 2018-2030 (USD MILLION)
TABLE 52. GLOBAL DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY END USER, 2018-2030 (USD MILLION)
TABLE 53. GLOBAL DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY DIAGNOSTIC LABORATORIES, BY REGION, 2018-2030 (USD MILLION)
TABLE 54. GLOBAL DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY DIAGNOSTIC LABORATORIES, BY GROUP, 2018-2030 (USD MILLION)
TABLE 55. GLOBAL DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY DIAGNOSTIC LABORATORIES, BY COUNTRY, 2018-2030 (USD MILLION)
TABLE 56. GLOBAL DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY HOME CARE SETTINGS, BY REGION, 2018-2030 (USD MILLION)
TABLE 57. GLOBAL DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY HOME CARE SETTINGS, BY GROUP, 2018-2030 (USD MILLION)
TABLE 58. GLOBAL DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY HOME CARE SETTINGS, BY COUNTRY, 2018-2030 (USD MILLION)
TABLE 59. GLOBAL DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY HOSPITALS & CLINICS, BY REGION, 2018-2030 (USD MILLION)
TABLE 60. GLOBAL DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY HOSPITALS & CLINICS, BY GROUP, 2018-2030 (USD MILLION)
TABLE 61. GLOBAL DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY HOSPITALS & CLINICS, BY COUNTRY, 2018-2030 (USD MILLION)
TABLE 62. GLOBAL DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY RESEARCH INSTITUTES, BY REGION, 2018-2030 (USD MILLION)
TABLE 63. GLOBAL DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY RESEARCH INSTITUTES, BY GROUP, 2018-2030 (USD MILLION)
TABLE 64. GLOBAL DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY RESEARCH INSTITUTES, BY COUNTRY, 2018-2030 (USD MILLION)
TABLE 65. GLOBAL DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY DISTRIBUTION CHANNEL, 2018-2030 (USD MILLION)
TABLE 66. GLOBAL DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY DIRECT SALES, BY REGION, 2018-2030 (USD MILLION)
TABLE 67. GLOBAL DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY DIRECT SALES, BY GROUP, 2018-2030 (USD MILLION)
TABLE 68. GLOBAL DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY DIRECT SALES, BY COUNTRY, 2018-2030 (USD MILLION)
TABLE 69. GLOBAL DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY DISTRIBUTORS & WHOLESALERS, BY REGION, 2018-2030 (USD MILLION)
TABLE 70. GLOBAL DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY DISTRIBUTORS & WHOLESALERS, BY GROUP, 2018-2030 (USD MILLION)
TABLE 71. GLOBAL DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY DISTRIBUTORS & WHOLESALERS, BY COUNTRY, 2018-2030 (USD MILLION)
TABLE 72. GLOBAL DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY REGION, 2018-2030 (USD MILLION)
TABLE 73. AMERICAS DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY SUBREGION, 2018-2030 (USD MILLION)
TABLE 74. AMERICAS DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY PRODUCT TYPE, 2018-2030 (USD MILLION)
TABLE 75. AMERICAS DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY TECHNOLOGY, 2018-2030 (USD MILLION)
TABLE 76. AMERICAS DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY SAMPLE TYPE, 2018-2030 (USD MILLION)
TABLE 77. AMERICAS DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY APPLICATION, 2018-2030 (USD MILLION)
TABLE 78. AMERICAS DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY NEWBORN SCREENING, 2018-2030 (USD MILLION)
TABLE 79. AMERICAS DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY END USER, 2018-2030 (USD MILLION)
TABLE 80. AMERICAS DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY DISTRIBUTION CHANNEL, 2018-2030 (USD MILLION)
TABLE 81. NORTH AMERICA DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY COUNTRY, 2018-2030 (USD MILLION)
TABLE 82. NORTH AMERICA DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY PRODUCT TYPE, 2018-2030 (USD MILLION)
TABLE 83. NORTH AMERICA DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY TECHNOLOGY, 2018-2030 (USD MILLION)
TABLE 84. NORTH AMERICA DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY SAMPLE TYPE, 2018-2030 (USD MILLION)
TABLE 85. NORTH AMERICA DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY APPLICATION, 2018-2030 (USD MILLION)
TABLE 86. NORTH AMERICA DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY NEWBORN SCREENING, 2018-2030 (USD MILLION)
TABLE 87. NORTH AMERICA DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY END USER, 2018-2030 (USD MILLION)
TABLE 88. NORTH AMERICA DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY DISTRIBUTION CHANNEL, 2018-2030 (USD MILLION)
TABLE 89. LATIN AMERICA DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY COUNTRY, 2018-2030 (USD MILLION)
TABLE 90. LATIN AMERICA DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY PRODUCT TYPE, 2018-2030 (USD MILLION)
TABLE 91. LATIN AMERICA DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY TECHNOLOGY, 2018-2030 (USD MILLION)
TABLE 92. LATIN AMERICA DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY SAMPLE TYPE, 2018-2030 (USD MILLION)
TABLE 93. LATIN AMERICA DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY APPLICATION, 2018-2030 (USD MILLION)
TABLE 94. LATIN AMERICA DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY NEWBORN SCREENING, 2018-2030 (USD MILLION)
TABLE 95. LATIN AMERICA DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY END USER, 2018-2030 (USD MILLION)
TABLE 96. LATIN AMERICA DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY DISTRIBUTION CHANNEL, 2018-2030 (USD MILLION)
TABLE 97. EUROPE, MIDDLE EAST & AFRICA DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY SUBREGION, 2018-2030 (USD MILLION)
TABLE 98. EUROPE, MIDDLE EAST & AFRICA DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY PRODUCT TYPE, 2018-2030 (USD MILLION)
TABLE 99. EUROPE, MIDDLE EAST & AFRICA DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY TECHNOLOGY, 2018-2030 (USD MILLION)
TABLE 100. EUROPE, MIDDLE EAST & AFRICA DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY SAMPLE TYPE, 2018-2030 (USD MILLION)
TABLE 101. EUROPE, MIDDLE EAST & AFRICA DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY APPLICATION, 2018-2030 (USD MILLION)
TABLE 102. EUROPE, MIDDLE EAST & AFRICA DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY NEWBORN SCREENING, 2018-2030 (USD MILLION)
TABLE 103. EUROPE, MIDDLE EAST & AFRICA DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY END USER, 2018-2030 (USD MILLION)
TABLE 104. EUROPE, MIDDLE EAST & AFRICA DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY DISTRIBUTION CHANNEL, 2018-2030 (USD MILLION)
TABLE 105. EUROPE DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY COUNTRY, 2018-2030 (USD MILLION)
TABLE 106. EUROPE DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY PRODUCT TYPE, 2018-2030 (USD MILLION)
TABLE 107. EUROPE DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY TECHNOLOGY, 2018-2030 (USD MILLION)
TABLE 108. EUROPE DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY SAMPLE TYPE, 2018-2030 (USD MILLION)
TABLE 109. EUROPE DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY APPLICATION, 2018-2030 (USD MILLION)
TABLE 110. EUROPE DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY NEWBORN SCREENING, 2018-2030 (USD MILLION)
TABLE 111. EUROPE DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY END USER, 2018-2030 (USD MILLION)
TABLE 112. EUROPE DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY DISTRIBUTION CHANNEL, 2018-2030 (USD MILLION)
TABLE 113. MIDDLE EAST DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY COUNTRY, 2018-2030 (USD MILLION)
TABLE 114. MIDDLE EAST DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY PRODUCT TYPE, 2018-2030 (USD MILLION)
TABLE 115. MIDDLE EAST DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY TECHNOLOGY, 2018-2030 (USD MILLION)
TABLE 116. MIDDLE EAST DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY SAMPLE TYPE, 2018-2030 (USD MILLION)
TABLE 117. MIDDLE EAST DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY APPLICATION, 2018-2030 (USD MILLION)
TABLE 118. MIDDLE EAST DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY NEWBORN SCREENING, 2018-2030 (USD MILLION)
TABLE 119. MIDDLE EAST DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY END USER, 2018-2030 (USD MILLION)
TABLE 120. MIDDLE EAST DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY DISTRIBUTION CHANNEL, 2018-2030 (USD MILLION)
TABLE 121. AFRICA DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY COUNTRY, 2018-2030 (USD MILLION)
TABLE 122. AFRICA DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY PRODUCT TYPE, 2018-2030 (USD MILLION)
TABLE 123. AFRICA DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY TECHNOLOGY, 2018-2030 (USD MILLION)
TABLE 124. AFRICA DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY SAMPLE TYPE, 2018-2030 (USD MILLION)
TABLE 125. AFRICA DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY APPLICATION, 2018-2030 (USD MILLION)
TABLE 126. AFRICA DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY NEWBORN SCREENING, 2018-2030 (USD MILLION)
TABLE 127. AFRICA DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY END USER, 2018-2030 (USD MILLION)
TABLE 128. AFRICA DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY DISTRIBUTION CHANNEL, 2018-2030 (USD MILLION)
TABLE 129. ASIA-PACIFIC DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY COUNTRY, 2018-2030 (USD MILLION)
TABLE 130. ASIA-PACIFIC DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY PRODUCT TYPE, 2018-2030 (USD MILLION)
TABLE 131. ASIA-PACIFIC DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY TECHNOLOGY, 2018-2030 (USD MILLION)
TABLE 132. ASIA-PACIFIC DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY SAMPLE TYPE, 2018-2030 (USD MILLION)
TABLE 133. ASIA-PACIFIC DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY APPLICATION, 2018-2030 (USD MILLION)
TABLE 134. ASIA-PACIFIC DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY NEWBORN SCREENING, 2018-2030 (USD MILLION)
TABLE 135. ASIA-PACIFIC DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY END USER, 2018-2030 (USD MILLION)
TABLE 136. ASIA-PACIFIC DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY DISTRIBUTION CHANNEL, 2018-2030 (USD MILLION)
TABLE 137. GLOBAL DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY GROUP, 2018-2030 (USD MILLION)
TABLE 138. ASEAN DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY COUNTRY, 2018-2030 (USD MILLION)
TABLE 139. ASEAN DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY PRODUCT TYPE, 2018-2030 (USD MILLION)
TABLE 140. ASEAN DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY TECHNOLOGY, 2018-2030 (USD MILLION)
TABLE 141. ASEAN DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY SAMPLE TYPE, 2018-2030 (USD MILLION)
TABLE 142. ASEAN DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY APPLICATION, 2018-2030 (USD MILLION)
TABLE 143. ASEAN DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY NEWBORN SCREENING, 2018-2030 (USD MILLION)
TABLE 144. ASEAN DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY END USER, 2018-2030 (USD MILLION)
TABLE 145. ASEAN DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY DISTRIBUTION CHANNEL, 2018-2030 (USD MILLION)
TABLE 146. GCC DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY COUNTRY, 2018-2030 (USD MILLION)
TABLE 147. GCC DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY PRODUCT TYPE, 2018-2030 (USD MILLION)
TABLE 148. GCC DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY TECHNOLOGY, 2018-2030 (USD MILLION)
TABLE 149. GCC DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY SAMPLE TYPE, 2018-2030 (USD MILLION)
TABLE 150. GCC DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY APPLICATION, 2018-2030 (USD MILLION)
TABLE 151. GCC DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY NEWBORN SCREENING, 2018-2030 (USD MILLION)
TABLE 152. GCC DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY END USER, 2018-2030 (USD MILLION)
TABLE 153. GCC DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY DISTRIBUTION CHANNEL, 2018-2030 (USD MILLION)
TABLE 154. EUROPEAN UNION DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY COUNTRY, 2018-2030 (USD MILLION)
TABLE 155. EUROPEAN UNION DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY PRODUCT TYPE, 2018-2030 (USD MILLION)
TABLE 156. EUROPEAN UNION DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY TECHNOLOGY, 2018-2030 (USD MILLION)
TABLE 157. EUROPEAN UNION DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY SAMPLE TYPE, 2018-2030 (USD MILLION)
TABLE 158. EUROPEAN UNION DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY APPLICATION, 2018-2030 (USD MILLION)
TABLE 159. EUROPEAN UNION DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY NEWBORN SCREENING, 2018-2030 (USD MILLION)
TABLE 160. EUROPEAN UNION DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY END USER, 2018-2030 (USD MILLION)
TABLE 161. EUROPEAN UNION DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY DISTRIBUTION CHANNEL, 2018-2030 (USD MILLION)
TABLE 162. BRICS DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY COUNTRY, 2018-2030 (USD MILLION)
TABLE 163. BRICS DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY PRODUCT TYPE, 2018-2030 (USD MILLION)
TABLE 164. BRICS DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY TECHNOLOGY, 2018-2030 (USD MILLION)
TABLE 165. BRICS DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY SAMPLE TYPE, 2018-2030 (USD MILLION)
TABLE 166. BRICS DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY APPLICATION, 2018-2030 (USD MILLION)
TABLE 167. BRICS DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY NEWBORN SCREENING, 2018-2030 (USD MILLION)
TABLE 168. BRICS DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY END USER, 2018-2030 (USD MILLION)
TABLE 169. BRICS DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY DISTRIBUTION CHANNEL, 2018-2030 (USD MILLION)
TABLE 170. G7 DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY COUNTRY, 2018-2030 (USD MILLION)
TABLE 171. G7 DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY PRODUCT TYPE, 2018-2030 (USD MILLION)
TABLE 172. G7 DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY TECHNOLOGY, 2018-2030 (USD MILLION)
TABLE 173. G7 DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY SAMPLE TYPE, 2018-2030 (USD MILLION)
TABLE 174. G7 DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY APPLICATION, 2018-2030 (USD MILLION)
TABLE 175. G7 DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY NEWBORN SCREENING, 2018-2030 (USD MILLION)
TABLE 176. G7 DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY END USER, 2018-2030 (USD MILLION)
TABLE 177. G7 DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY DISTRIBUTION CHANNEL, 2018-2030 (USD MILLION)
TABLE 178. NATO DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY COUNTRY, 2018-2030 (USD MILLION)
TABLE 179. NATO DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY PRODUCT TYPE, 2018-2030 (USD MILLION)
TABLE 180. NATO DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY TECHNOLOGY, 2018-2030 (USD MILLION)
TABLE 181. NATO DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY SAMPLE TYPE, 2018-2030 (USD MILLION)
TABLE 182. NATO DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY APPLICATION, 2018-2030 (USD MILLION)
TABLE 183. NATO DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY NEWBORN SCREENING, 2018-2030 (USD MILLION)
TABLE 184. NATO DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY END USER, 2018-2030 (USD MILLION)
TABLE 185. NATO DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY DISTRIBUTION CHANNEL, 2018-2030 (USD MILLION)
TABLE 186. GLOBAL DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY COUNTRY, 2018-2030 (USD MILLION)
TABLE 187. UNITED STATES DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, 2018-2030 (USD MILLION)
TABLE 188. UNITED STATES DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY PRODUCT TYPE, 2018-2030 (USD MILLION)
TABLE 189. UNITED STATES DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY TECHNOLOGY, 2018-2030 (USD MILLION)
TABLE 190. UNITED STATES DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY SAMPLE TYPE, 2018-2030 (USD MILLION)
TABLE 191. UNITED STATES DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY APPLICATION, 2018-2030 (USD MILLION)
TABLE 192. UNITED STATES DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY NEWBORN SCREENING, 2018-2030 (USD MILLION)
TABLE 193. UNITED STATES DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY END USER, 2018-2030 (USD MILLION)
TABLE 194. UNITED STATES DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY DISTRIBUTION CHANNEL, 2018-2030 (USD MILLION)
TABLE 195. CHINA DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, 2018-2030 (USD MILLION)
TABLE 196. CHINA DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY PRODUCT TYPE, 2018-2030 (USD MILLION)
TABLE 197. CHINA DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY TECHNOLOGY, 2018-2030 (USD MILLION)
TABLE 198. CHINA DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY SAMPLE TYPE, 2018-2030 (USD MILLION)
TABLE 199. CHINA DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY APPLICATION, 2018-2030 (USD MILLION)
TABLE 200. CHINA DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY NEWBORN SCREENING, 2018-2030 (USD MILLION)
TABLE 201. CHINA DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY END USER, 2018-2030 (USD MILLION)
TABLE 202. CHINA DRIED BLOOD SPOT COLLECTION CARDS MARKET SIZE, BY DISTRIBUTION CHANNEL, 2018-2030 (USD MILLION)