Global Dried Blood Spot Collection Cards Market - Key Trends & Drivers Summarized
Is the Rising Focus on Remote Diagnostics and Decentralized Testing Throwing the Spotlight on Dried Blood Spot Collection Cards?
As healthcare systems worldwide pivot toward decentralized diagnostics and remote patient monitoring, dried blood spot (DBS) collection cards are gaining significant prominence as a minimally invasive, cost-effective, and highly portable biosampling method. DBS cards, which involve collecting small volumes of capillary blood on specialized filter paper, are increasingly used for diagnostic testing, disease surveillance, and therapeutic drug monitoring in both clinical and research settings. Their utility is particularly evident in resource-limited environments where traditional phlebotomy infrastructure is lacking or where cold-chain logistics are infeasible. From infectious disease testing (like HIV and hepatitis) to newborn screening, metabolic disorder detection, and pharmacokinetics, DBS cards are becoming an essential tool for accessible and scalable diagnostics. The COVID-19 pandemic further accelerated the demand for remote testing tools, placing renewed emphasis on DBS cards as part of home testing kits and public health surveillance initiatives. Their compatibility with mail-in diagnostics and ease of long-term sample storage without refrigeration make them especially useful for biobanking and longitudinal studies. As personalized medicine and epidemiological monitoring become more widespread, DBS collection cards are proving to be an efficient, patient-friendly method of enabling mass-scale testing with minimal infrastructure. This evolving demand landscape is positioning DBS cards as a core technology in the shift from centralized lab testing to decentralized, patient-centric healthcare models.How Are Technological Improvements Enhancing the Accuracy and Applications of DBS Collection Cards?
Innovations in sample collection materials, analytical sensitivity, and laboratory processing are driving the evolution of dried blood spot collection cards from simple field tools to precision diagnostic platforms. Modern DBS cards are manufactured using high-grade filter papers with uniform absorption, defined spot boundaries, and chemical stability that preserve analyte integrity over extended periods. Coated cards with chemical stabilizers or antimicrobial properties are being developed to support the safe collection and transport of volatile biomarkers, nucleic acids, or viral particles. These advancements have significantly expanded the analytical range of DBS cards, making them suitable not just for basic biomarker detection but also for genetic screening, molecular diagnostics, and next-generation sequencing (NGS) workflows. Automated punching systems and robotic sample handlers are reducing manual variability in labs, while improved extraction techniques are enabling the recovery of higher-quality analytes from smaller sample volumes. Laboratories can now perform highly sensitive assays - such as LC-MS/MS, ELISA, PCR, and even whole-genome analysis - using samples derived from DBS cards. Digital traceability solutions, including QR codes and cloud-based sample tracking, are also being integrated to enhance sample management and patient-data linkage. These technological upgrades are expanding the utility of DBS cards in clinical trials, drug development, and public health programs, positioning them as powerful tools not only for field diagnostics but also for precision laboratory-based applications.Is the Expansion of At-Home Testing and Population Screening Boosting Demand for DBS Cards?
The global rise of consumer-directed health testing, at-home diagnostics, and national screening initiatives is creating fertile ground for the expansion of dried blood spot collection card usage. Consumers today are more willing to participate in health monitoring outside traditional clinical settings, driven by convenience, privacy, and the digital health ecosystem’ s rapid growth. At-home testing services - offering everything from cholesterol checks to food sensitivity panels and hormone level assessments - are increasingly adopting DBS collection kits for their simplicity and mail-ready format. National health agencies and NGOs are also leveraging DBS-based collection methods in large-scale population health studies, newborn screening programs, vaccination response monitoring, and epidemiological surveillance. The cards’ ability to retain sample integrity over time and under variable storage conditions makes them highly suitable for use in rural or underserved populations where medical infrastructure is limited. Moreover, in clinical trials and decentralized research models, DBS cards are streamlining participant enrollment and sample logistics by allowing remote collection and centralized analysis. Telemedicine platforms are integrating DBS kits into chronic disease management programs for diabetes, cardiovascular conditions, and HIV, enabling regular monitoring without in-person visits. The rising trust in self-administered diagnostic tools, coupled with advancements in digital health interfaces, is reinforcing the value proposition of DBS cards as critical enablers of accessible, scalable, and patient-driven diagnostics in both developed and emerging markets.What Are the Key Drivers Fueling Growth in the Dried Blood Spot Collection Cards Market?
The growth in the dried blood spot collection cards market is driven by a combination of technological, clinical, and behavioral factors shaping the future of diagnostics and biosampling. A primary growth driver is the widespread push toward decentralizing healthcare services, particularly in light of lessons learned during the COVID-19 pandemic, which highlighted the value of remote, low-contact diagnostic tools. Rising prevalence of chronic and infectious diseases is prompting broader use of DBS cards for regular monitoring and screening, especially in resource-constrained settings where access to phlebotomy is limited. The expansion of genetic and molecular testing is also increasing demand for high-quality biospecimens that can be collected easily and stored long-term without specialized refrigeration or transport systems. Government support for public health surveillance, maternal and child health programs, and national biobanking efforts is creating a robust institutional demand base for DBS collection systems. The growing popularity of direct-to-consumer health testing and personalized medicine is fueling adoption by private diagnostic firms and wellness platforms. Additionally, the reduced cost of collection, storage, and shipment relative to traditional blood draws makes DBS cards economically attractive for both clinical and research applications. Regulatory bodies in multiple regions are increasingly recognizing DBS as a valid specimen format for diagnostic and pharmacological use, further legitimizing its role in healthcare workflows. Together, these factors are accelerating the global adoption of DBS collection cards and establishing them as an essential tool in the future of decentralized and preventive healthcare.Report Scope
The report analyzes the Dried Blood Spot Collection Cards market, presented in terms of market value. The analysis covers the key segments and geographic regions outlined below.- Segments: Card Type (Whatman 903 Card, Ahlstrom 226 Card, FTA Card, Other Card Types); Application (New Born Screening Application, Infectious Diseases Testing Application, Therapeutic Drug Monitoring Application, Forensics Application, CRO / Research Application, Other Applications); End-Use (Hospitals & Clinics End-Use, Diagnostics Centers End-Use, Other End-Uses).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Whatman 903 Card segment, which is expected to reach US$197.1 Million by 2030 with a CAGR of a 4.3%. The Ahlstrom 226 Card segment is also set to grow at 2.2% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $105.6 Million in 2024, and China, forecasted to grow at an impressive 6.2% CAGR to reach $93.5 Million by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Dried Blood Spot Collection Cards Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Dried Blood Spot Collection Cards Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Dried Blood Spot Collection Cards Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Aachen Akti Navigation Company, Abeko Dredging & Marine Contractors, Adani Ports and Special Economic Zone, Aquarius Systems, Baggerbedrijf de Boer - Dutch Dredging and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 36 companies featured in this Dried Blood Spot Collection Cards market report include:
- Ahlstrom-Munksjö
- ARCHIMEDlife
- CENTOGENE N.V.
- Eastern Business Forms, Inc.
- F. Hoffmann-La Roche Ltd
- Gentegra LLC
- Hemaxis
- LaCAR MDx
- Lipomic Healthcare
- Metabolon
- Neoteryx
- PerkinElmer
- QIAGEN
- RDA Spot
- Shimadzu Scientific Instruments
- Synergy Medical Systems LLP
- Thomas Scientific
- Trajan Scientific Australia Pty Ltd
- Whatman
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Ahlstrom-Munksjö
- ARCHIMEDlife
- CENTOGENE N.V.
- Eastern Business Forms, Inc.
- F. Hoffmann-La Roche Ltd
- Gentegra LLC
- Hemaxis
- LaCAR MDx
- Lipomic Healthcare
- Metabolon
- Neoteryx
- PerkinElmer
- QIAGEN
- RDA Spot
- Shimadzu Scientific Instruments
- Synergy Medical Systems LLP
- Thomas Scientific
- Trajan Scientific Australia Pty Ltd
- Whatman
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 381 |
| Published | December 2025 |
| Forecast Period | 2024 - 2030 |
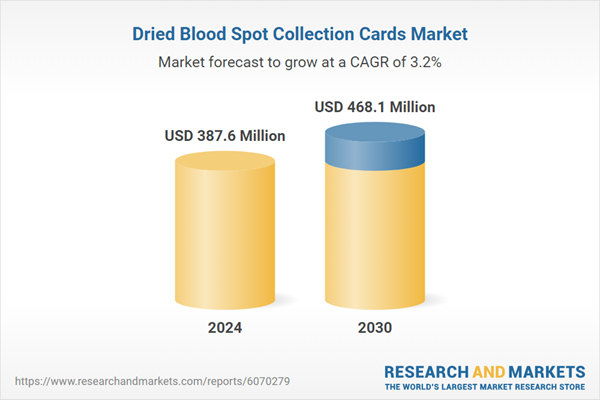
| Estimated Market Value ( USD | $ 387.6 Million |
| Forecasted Market Value ( USD | $ 468.1 Million |
| Compound Annual Growth Rate | 3.2% |
| Regions Covered | Global |