Speak directly to the analyst to clarify any post sales queries you may have.
Introduction to the emerging convergence of clinical need, ingredient innovation, and distribution evolution shaping modern dysphagia supplement development
The landscape for dysphagia supplements is evolving at the intersection of clinical need, nutritional science, and consumer-driven convenience. As clinicians refine diagnostic pathways and caregivers seek evidence-based interventions, nutritional products designed for individuals with swallowing difficulties are transitioning from niche therapeutic adjuncts to integral components of multidisciplinary care. Innovations in texture modification, solubility, and palatability are enabling formulations that meet both clinical safety criteria and patient acceptance, thereby improving adherence and therapeutic outcomes.Meanwhile, ingredient science is advancing in parallel, with renewed attention on the functional properties of carbohydrates, enzymes, fibers, proteins, and targeted micronutrient fortification. These developments are creating a more nuanced product taxonomy that addresses therapeutic endpoints such as rehabilitation support, aspiration risk mitigation, and maintenance of nutritional status. At the same time, distribution channels and care settings-from hospitals and long-term care facilities to home-based management supported by online retail platforms-are reshaping how these products are accessed and administered.
Regulatory frameworks and labeling expectations are also exerting influence, prompting manufacturers to invest in clinical validation and traceability. In combination, these forces are driving a move toward integrated solutions that blend clinical rigor with consumer-centric design. Consequently, stakeholders across clinical, manufacturing, and commercial domains must align product development and go-to-market strategies to meet evolving standards of care and caregiver expectations.
Transformative shifts driven by clinical evidence, formulation breakthroughs, and distribution channel advancement that are reshaping dysphagia supplement strategies
Recent transformative shifts are redefining the competitive and clinical contours of the dysphagia supplements sector, with a series of interlocking dynamics accelerating innovation. First, clinical pathways are becoming more data-driven: improved screening tools and interdisciplinary care models are identifying candidates for targeted nutritional interventions earlier, which in turn increases demand for products that integrate clinical evidence with user-centered design. Concurrently, advances in formulation science-such as enzyme-assisted texture modification and protein matrix engineering-are enabling products that reconcile safety, nutritional density, and sensory acceptability, thereby addressing long-standing adherence barriers.On the commercial side, distribution paradigms are shifting as digital channels mature and institutional procurement practices adapt to value-based care priorities. Enhanced online platforms are improving access for home-based users while pharmacy and hospital procurement teams emphasize supplier reliability and product traceability. In addition, regulatory scrutiny and evolving labelling expectations are prompting companies to strengthen clinical substantiation and quality systems, which is favoring firms with integrated R&D and robust compliance functions. Sustainability and supply chain transparency have also emerged as differentiators, influencing ingredient sourcing and packaging decisions.
Taken together, these shifts are compelling established manufacturers and emerging entrants to pursue cross-disciplinary collaborations, invest in clinical evidence generation, and optimize omnichannel strategies that align with the needs of clinicians, caregivers, and patients.
Analysis of how recent tariff policy adjustments have reshaped ingredient sourcing, supply chain resilience, and commercial pricing strategies across the sector
Policy changes affecting tariffs and trade have introduced new cost dynamics and strategic considerations for companies operating in the dysphagia supplements ecosystem. The cumulative impact of tariff adjustments has reverberated across ingredient procurement, packaging imports, and manufacturing inputs, prompting procurement teams to reassess supplier footprints and cost pass-through strategies. In practice, reliance on imported specialty ingredients such as certain enzyme preparations, protein isolates, and advanced excipients has highlighted supply chain vulnerability, encouraging manufacturers to explore alternate sourcing and supplier qualification pathways.In response, many stakeholders have intensified efforts to diversify supplier networks and to evaluate nearshoring opportunities that reduce tariff exposure and compress lead times. At the same time, manufacturers and distributors are reconfiguring cost structures by optimizing packaging designs, rationalizing SKU complexity, and negotiating longer-term agreements with strategic suppliers to stabilize input pricing. Regulatory compliance and customs classification have become more prominent operational priorities, as misclassification can exacerbate tariff liabilities and delay shipments to clinical and retail channels.
Moreover, tariff-driven cost pressures have influenced pricing strategies and commercial contracting, particularly with institutional buyers and large pharmacy chains that demand predictable margins and service levels. As a result, companies are deploying multifaceted mitigation tactics-ranging from product reformulation to vertical integration of critical inputs-to preserve competitiveness while maintaining the clinical and sensory attributes that define acceptable dysphagia products.
Comprehensive segmentation-driven insights across product form, end user, distribution channel, ingredient type, application, and age group for precise strategic targeting
A nuanced segmentation framework reveals how product development, commercialization, and clinical use vary across distinct axes. Based on Product Form, market dynamics differ substantially among Capsule, Gel, Liquid, Powder, and Tablet formats, with each form presenting unique formulation challenges related to texture, dissolution behavior, and dosing accuracy. Capsules and tablets prioritize stability and dosing precision, whereas gels, liquids, and powders emphasize rheological control and sensory optimization for safe swallowing. Based on End User, adoption patterns are shaped by the needs of Home Care, Hospitals, Long-Term Care Facilities, Online Retail, and Pharmacies; importantly, online retail is further subdivided into Company Websites and E-Commerce Marketplaces, which necessitates distinct fulfillment and marketing approaches tailored to caregiver research behaviors and regulatory compliance for direct-to-consumer channels.Based on Distribution Channel, product availability and margin structures vary across E-Commerce Platforms, Grocery & Specialty Stores, Hospital Pharmacy, Online Pharmacy, and Retail Pharmacy, with E-Commerce Platforms further studied across Amazon as a prominent marketplace actor that influences discoverability and logistics expectations. Based on Ingredient Type, product positioning and therapeutic claims map to Carbohydrate-Based, Enzyme-Based, Fiber-Based, Protein-Based, and Vitamin & Mineral Fortified formulations. Within Carbohydrate-Based ingredients, sub-classifications such as Dextrin, Maltodextrin, and Starch inform viscosity and solubility engineering, while the Fiber-Based category differentiates Insoluble and Soluble options that affect mouthfeel and gastrointestinal tolerance. The Protein-Based segment includes Casein, Pea, Soy, and Whey, each bringing different allergen profiles, amino acid composition, and functional properties, and the Vitamin & Mineral Fortified category spans Mineral Fortified, Multivitamin, and Single Vitamin approaches that target micronutrient replenishment.
Based on Application, product development splits between Preventive and Therapeutic use cases: Preventive applications emphasize General Wellness and Maintenance, while Therapeutic formulations address Dysphagia Rehabilitation and Nutritional Support with clinical endpoints in mind. Finally, Based on Age Group, formulation preferences and regulatory considerations vary across Adult, Geriatric, and Pediatric cohorts; the Adult segment further differentiates 18-44 and 45-64 subgroups, the Geriatric group separates 65-74 and 75+ cohorts, and the Pediatric population is disaggregated into Adolescents, Children, and Infants, each requiring tailored dosing, flavoring, and safety profiles. This segmentation underscores the imperative for product portfolios that are both granular and clinically aligned, enabling companies to target specific clinical needs and channel dynamics effectively.
Regional dynamics and differentiated go-to-market approaches across Americas, Europe Middle East & Africa, and Asia-Pacific that determine local adoption and supply strategies
Regional dynamics exert strong influence on ingredient sourcing, regulatory pathways, and commercial models. In the Americas, demographics and institutional care infrastructures support robust clinical adoption, while e-commerce acceleration and pharmacy networks create diverse access routes for home-based users. Manufacturers operating in the Americas often prioritize compliance with region-specific labeling and clinical substantiation standards, and they typically invest in partnerships with hospital systems and long-term care providers to establish formulary placement and clinician trust.In Europe, Middle East & Africa, regulatory heterogeneity and reimbursement variability drive the need for localized market strategies that account for national healthcare protocols and procurement practices. Innovation adoption in this combined region often hinges on demonstrated clinical outcomes and alignment with institutional formularies, and companies frequently collaborate with local distributors to navigate complex import requirements and varied caregiver expectations. Meanwhile, Asia-Pacific presents a mix of rapid consumer adoption in urban markets and divergent clinical infrastructures across countries; the region’s manufacturing capacity and established ingredients supply chains make it an attractive base for scale production, while rising digital penetration accelerates direct-to-consumer access to therapeutic nutrition products.
Across all regions, cross-border supply chain decisions, local regulatory interpretation, and channel preferences shape product design and commercialization prioritization. Consequently, a regionalized go-to-market approach that balances centralized R&D efficiencies with localized regulatory and caregiver engagement is critical for sustained adoption and long-term growth.
How leading players are combining clinical evidence, supply chain control, and strategic partnerships to differentiate product portfolios and capture institutional trust
Competitive positioning among leading companies reflects a dual focus on clinical evidence generation and operational scale. Established manufacturers are investing in clinical trials, real-world evidence programs, and rigorous quality systems to substantiate safety and efficacy claims, thereby strengthening relationships with hospital procurement teams and long-term care administrators. At the same time, nimble entrants are differentiating through rapid product iteration, emphasis on sensory experience, and targeted digital marketing to caregivers and home users. Strategic partnerships between ingredient suppliers, contract manufacturers, and clinical research organizations are becoming more prevalent as firms seek to accelerate innovation while managing capital intensity.Vertical integration of critical inputs, whether through closer supplier relationships or selective investment in production capabilities, is enabling companies to mitigate supply chain risk and enhance margin predictability. Meanwhile, convergence between medical device companies and nutrition brands is emerging as a pathway to deliver integrated dysphagia management solutions that combine assessment tools with tailored supplements. Companies that prioritize interoperability with clinical workflows and invest in training resources for healthcare professionals tend to achieve greater acceptance in institutional settings. Finally, brand trust and transparent communication of clinical data are central to differentiation, especially when navigating sensitive populations such as geriatrics and pediatrics where safety and tolerability drive purchasing decisions.
Practical and prioritized strategic moves for manufacturers and distributors to strengthen clinical credibility, supply resilience, and go-to-market effectiveness
Industry leaders should pursue a set of pragmatic, action-oriented strategies to consolidate clinical credibility and operational resilience. First, prioritize clinical evidence generation that addresses both safety in swallowing mechanics and longer-term nutritional outcomes; partnering with academic centers and clinical networks can accelerate robust studies and facilitate guideline inclusion. Second, diversify ingredient sourcing and qualify secondary suppliers for critical inputs such as specialty enzymes and protein isolates to reduce tariff exposure and supply disruption risk. Third, invest in formulation capabilities that span texture-modified gels and easily-dissolved powders while maintaining palatability and dosing accuracy to enhance adherence across age groups.Fourth, align channel strategies by developing tailored value propositions for hospitals, long-term care facilities, pharmacies, and digital retailers; this includes optimizing packaging and fulfillment for direct-to-consumer purchases while ensuring compliance with institutional procurement requirements. Fifth, deepen engagement with clinicians and caregivers through education programs, training modules, and point-of-care resources that translate product features into clinical benefits. Sixth, implement pricing and contracting models that reflect institutional purchasing cycles and that offer predictable total cost of care outcomes. Seventh, embed sustainability and traceability into sourcing and packaging decisions to meet rising stakeholder expectations. Finally, leverage digital analytics to refine targeting, measure outcomes, and iterate product positioning based on real-world feedback from both clinical and home settings.
A transparent and rigorous mixed-methods research approach integrating primary expert interviews, technical consultations, and secondary evidence for defensible insights
The research underpinning this report synthesizes primary and secondary methods to ensure robust, triangulated insights. Primary research included structured interviews with clinicians, procurement professionals, caregivers, and manufacturing experts to capture first-hand perspectives on clinical needs, product performance, and channel preferences. Supplementing these interviews, technical consultations with formulation scientists and supply chain managers provided detailed understanding of ingredient behavior, processing constraints, and quality control priorities. Secondary research encompassed peer-reviewed clinical literature, regulatory guidance documents, product labels, and patent landscapes to contextualize claims and identify innovation trends.Data triangulation methods were applied to reconcile qualitative findings with documented scientific evidence and regulatory parameters, enhancing the validity of conclusions. Segmentation and regional analyses were developed through iterative synthesis of stakeholder inputs and documented supply chain flows, with attention to regulatory divergence and channel structures. The methodology emphasizes transparency: assumptions and data sources are documented, and methodological limitations are acknowledged, including variability in clinical practices across geographies and evolving policy contexts. Where applicable, sensitivity checks and scenario analyses were performed to test the robustness of strategic implications, and findings were refined through internal peer review and expert validation.
Conclusion synthesizing clinical, formulation, and commercial imperatives that determine which organizations will lead in delivering safe and effective dysphagia nutrition solutions
In conclusion, the dysphagia supplements landscape is characterized by accelerating clinical integration, formulation innovation, and shifting commercial channels that collectively demand proactive strategic responses. Companies that combine rigorous clinical validation with adaptable formulation technologies will be best positioned to meet the diverse needs of hospitals, long-term care facilities, home-based users, and digital consumers. Furthermore, supply chain resilience and regulatory agility are essential given evolving trade policies and the criticality of specialty ingredients.Strategic differentiation will depend on the ability to translate clinical evidence into trusted products while optimizing distribution strategies for the specific demands of institutional and consumer channels. By adopting a segmented approach across product form, ingredient type, application, and age group, organizations can target development and commercialization efforts more effectively. Ultimately, stakeholders that integrate clinical partnerships, robust quality systems, and nimble commercial execution will secure the most meaningful impact in improving outcomes for individuals with swallowing challenges.
Additional Product Information:
- Purchase of this report includes 1 year online access with quarterly updates.
- This report can be updated on request. Please contact our Customer Experience team using the Ask a Question widget on our website.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
18. China Dysphagia Supplements Market
Companies Mentioned
The key companies profiled in this Dysphagia Supplements market report include:- Abbott Laboratories
- Agro Gums
- Altrafine Gums
- Ascot Pharma Ltd.
- AYMES International Ltd.
- Clinico Co., Ltd.
- Danone S.A.
- Flavour Creations
- Foricafoods corporation
- Fresenius Kabi AG
- Healthy Food Co., Ltd.
- Hormel Foods Corporation
- Kent Precision Foods Group, Inc.
- Kewpie Corporation
- Kissei Pharmaceutical Co., Ltd.
- Matsun Nutrition
- Meiji Holdings Co., Ltd.
- Nestlé S.A.
- Nutri Co., Ltd.
- Nutrinovo Ltd.
- Rosemont Pharmaceuticals Limited
- Sanulac Nutritionals Australia Pty Ltd.
- SimplyThick, LLC
- Slo Drinks
- Trisco Foods Pty Ltd.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 184 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
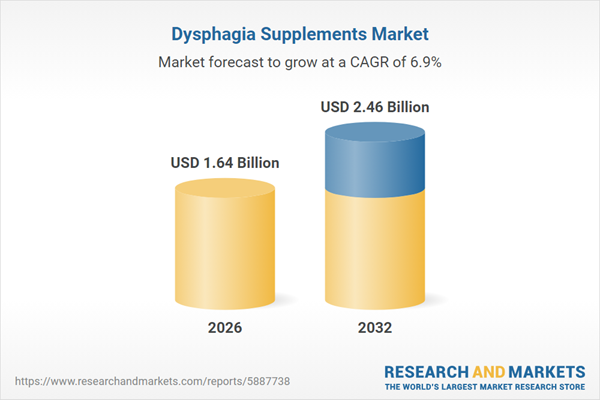
| Estimated Market Value ( USD | $ 1.64 Billion |
| Forecasted Market Value ( USD | $ 2.46 Billion |
| Compound Annual Growth Rate | 6.8% |
| Regions Covered | Global |
| No. of Companies Mentioned | 26 |