Global Ebola Virus Vaccines Market - Key Trends & Drivers Summarized
Why Is the Development of Ebola Virus Vaccines Still a Global Priority?
Ebola virus disease (EVD) remains one of the world's most lethal viral infections, with periodic outbreaks posing major public health threats in sub-Saharan Africa and beyond. The virus, part of the Filoviridae family, causes severe hemorrhagic fever with high fatality rates - often exceeding 50% - and has led to multiple epidemics over the past two decades, the most significant being the 2014-2016 West African outbreak. While outbreak containment through public health measures has improved, the high contagion rate and zoonotic nature of the virus continue to underscore the need for a robust global vaccine arsenal.The approval of vaccines like Merck's Ervebo (rVSV-ZEBOV) in 2019 was a milestone in the global response to Ebola. However, the virus's multiple strains - including Sudan and Bundibugyo variants - have driven the ongoing development of multivalent vaccines capable of broader protection. Recent flare-ups in Uganda and the Democratic Republic of Congo have reignited urgency among global health agencies and vaccine developers to expand stockpiles and diversify the vaccine pipeline. Organizations such as the WHO, CEPI, and Gavi are prioritizing vaccine accessibility and equitable deployment as part of the broader pandemic preparedness strategy.
What Are the Latest Advances in Ebola Vaccine Technology and Deployment?
Vaccine innovation for Ebola is progressing on multiple fronts. The leading licensed product, Ervebo, is a live attenuated, recombinant vaccine using vesicular stomatitis virus (VSV) as a vector, which provides fast-acting immunity - making it ideal for ring vaccination during outbreaks. Johnson & Johnson's two-dose Zabdeno/Mvabea regimen, utilizing adenovirus and MVA vectors, offers broader strain protection, albeit with a longer immunization timeline. These platforms are now being evaluated for expanded access use and cross-protection against related viruses.New candidates are being explored based on mRNA, DNA, and nanoparticle-based platforms, mirroring the technological success seen during COVID-19 vaccine development. Researchers are aiming to create thermostable vaccines suitable for use in remote, low-resource environments without stringent cold chain requirements. Additionally, needle-free delivery systems, such as intradermal patches and nasal sprays, are under preclinical investigation to support more scalable and field-friendly deployment in outbreak scenarios. These advancements reflect a global push to make Ebola vaccines not only effective but also practical for mass immunization efforts during emergencies.
How Are Global Health Systems and Partnerships Shaping Vaccine Accessibility?
Global public health agencies, governments, and NGOs are deeply involved in shaping Ebola vaccine distribution strategies. WHO and UNICEF have created emergency stockpiles of Ervebo and are working to ensure deployment logistics in outbreak zones are rapid and well-coordinated. Gavi, the Vaccine Alliance, is funding procurement and helping integrate Ebola vaccines into national preparedness plans in at-risk countries. Simultaneously, CEPI is funding next-generation candidates and aiming to establish a “100-day response” model for rapid scale-up in future outbreaks.Regulatory agencies are playing a proactive role in accelerating vaccine approvals through mechanisms like the WHO Emergency Use Listing (EUL), FDA Breakthrough Therapy Designation, and EMA PRIME scheme. In parallel, pharmaceutical companies are entering partnerships with local manufacturers and global health bodies to build regional production capacity and improve last-mile delivery. Despite these efforts, challenges remain - including vaccine hesitancy, political instability, and underdeveloped healthcare infrastructure - which require coordinated international responses to overcome.
What Is Driving Growth and Strategic Investment in the Ebola Vaccine Market?
The growth in the Ebola virus vaccines market is driven by several factors related to viral evolution, rising outbreak frequency, and advancements in vaccine platform technologies. The unpredictable resurgence of Ebola in endemic regions and the emergence of distinct viral strains have compelled the global health community to invest in more flexible, broad-spectrum vaccines. Technological progress in vector-based and mRNA vaccine platforms is enabling faster development cycles and improved adaptability for multivalent formulations. These innovations are supporting a shift from reactive outbreak response to proactive epidemic preparedness.End-use demand is also expanding due to increased focus on pre-exposure prophylaxis for frontline healthcare workers, military personnel, and humanitarian staff operating in high-risk areas. Government and donor funding, particularly from the U.S. BARDA, the EU Horizon program, and philanthropic sources like the Gates Foundation, is fueling vaccine R&D and stockpile expansion. Moreover, the integration of Ebola vaccine strategies into broader global health security frameworks - alongside COVID-19, Marburg virus, and other emerging pathogens - is ensuring long-term institutional support. These drivers collectively point to a sustained and strategically vital market trajectory for Ebola virus vaccines over the coming decade.
Report Scope
The report analyzes the Ebola Virus Vaccines market, presented in terms of market value (US$). The analysis covers the key segments and geographic regions outlined below:- Segments: Vaccine Type (cAd3-ZEBOV, rVSV-ZEBOV); Strain Type (Zaire, Sudan, Tai Forest, Reston, Bundibugyo Virus); Distribution Channel (Hospital Pharmacies, Retail Pharmacies, Online Pharmacies); End-Use (Hospitals, Homecare, Specialty Clinics, Ambulatory Surgery Centers, Other End-Uses).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the cAd3-ZEBOV Vaccine segment, which is expected to reach US$198.9 Million by 2030 with a CAGR of a 6.9%. The rVSV-ZEBOV Vaccine segment is also set to grow at 3.9% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $51.6 Million in 2024, and China, forecasted to grow at an impressive 9.8% CAGR to reach $55.6 Million by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Ebola Virus Vaccines Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Ebola Virus Vaccines Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Ebola Virus Vaccines Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 34 companies featured in this Ebola Virus Vaccines market report include:
- Bavarian Nordic A/S
- BioCryst Pharmaceuticals, Inc.
- Emergent BioSolutions Inc.
- GeoVax Labs, Inc.
- GlaxoSmithKline plc
- Immunovaccine Inc. (IMV Inc.)
- Inovio Pharmaceuticals, Inc.
- Johnson & Johnson
- Mapp Biopharmaceutical, Inc.
- Merck & Co., Inc.
- NewLink Genetics Corporation
- Novavax, Inc.
- Okairos AG
- Profectus BioSciences, Inc.
- Protein Sciences Corporation
- Sanofi S.A.
- Sarepta Therapeutics, Inc.
- Vaxart, Inc.
- VBI Vaccines Inc.
- Zydus Cadila
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Bavarian Nordic A/S
- BioCryst Pharmaceuticals, Inc.
- Emergent BioSolutions Inc.
- GeoVax Labs, Inc.
- GlaxoSmithKline plc
- Immunovaccine Inc. (IMV Inc.)
- Inovio Pharmaceuticals, Inc.
- Johnson & Johnson
- Mapp Biopharmaceutical, Inc.
- Merck & Co., Inc.
- NewLink Genetics Corporation
- Novavax, Inc.
- Okairos AG
- Profectus BioSciences, Inc.
- Protein Sciences Corporation
- Sanofi S.A.
- Sarepta Therapeutics, Inc.
- Vaxart, Inc.
- VBI Vaccines Inc.
- Zydus Cadila
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 336 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
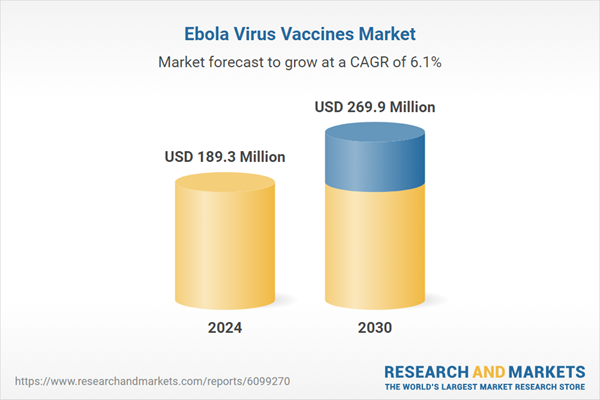
| Estimated Market Value ( USD | $ 189.3 Million |
| Forecasted Market Value ( USD | $ 269.9 Million |
| Compound Annual Growth Rate | 6.1% |
| Regions Covered | Global |