Speak directly to the analyst to clarify any post sales queries you may have.
Positioning endosurgery devices at the intersection of clinical innovation, operational efficiency, and tightened regulatory expectations across care delivery settings
A clear-sighted introduction that frames endosurgery devices within contemporary clinical practice and innovation dynamics
Endosurgery devices have moved from niche procedural aids to central enablers of minimally invasive care across multiple specialties. Advances in optics, energy delivery, and instrument ergonomics are reshaping procedural workflows, driving greater procedural precision while minimizing patient trauma. These technological shifts are occurring alongside changes in care delivery models, with procedures increasingly migrating out of traditional operating rooms into ambulatory surgical centers and specialized clinics that prioritize throughput, cost-efficiency, and rapid patient turnover. Clinicians and health systems are therefore recalibrating procurement criteria to emphasize device interoperability, ease of use, and total cost of ownership rather than simple unit price.Concurrently, regulatory scrutiny around safety, reprocessing, and device lifecycle management has intensified, prompting manufacturers to accelerate design for safety and to offer clearer guidance on use and maintenance. The confluence of these factors is shaping product development priorities and influencing where investment capital flows. As purchasers, providers, and innovators respond to these pressures, strategic clarity about clinical value propositions, supply chain resilience, and commercialization pathways becomes essential for market participants aiming to sustain long-term growth and clinical adoption
How technological convergence, shifting care pathways, and resilient supply chain strategies are driving a new era for endosurgery device innovation and adoption
Transformative shifts in clinical practices, technology convergence, and supply chain architectures that are redefining the endosurgery device landscape
Recent years have seen a convergence of imaging, energy, and digital technologies that is accelerating the evolution of endosurgery. High-resolution visualization platforms paired with advanced energy modalities enable clinicians to perform more complex interventions with minimally invasive approaches. Digitization, including integrated image guidance and procedural analytics, is enhancing intraoperative decision-making and enabling post-procedure performance tracking. These technology-driven gains are mirrored by changing care pathways, as hospitals and ambulatory sites pursue value-based care imperatives that reward better clinical outcomes and shorter stays.Parallel to technological evolution, supply chain strategies have shifted from just-in-time inventory models toward greater emphasis on resilience and nearshoring. Disruptions in global logistics have prompted providers and manufacturers to reassess sourcing strategies, increase safety stock for critical components, and diversify supplier portfolios. Regulatory developments also exert a powerful influence, with authorities focusing on device safety, traceability, and standardized reprocessing practices. The resulting environment favors manufacturers who can marry clinical innovation with robust quality systems and flexible manufacturing capabilities, enabling rapid response to both clinical needs and regulatory change
Understanding the practical implications of 2025 United States tariff revisions on procurement, manufacturing localization, and supply chain resiliency for endosurgery devices
Assessing the cumulative operational, procurement, and strategic consequences of United States tariff changes implemented in 2025 on endosurgery device stakeholders
Tariff adjustments introduced in 2025 have had ripple effects across the endosurgery value chain, influencing procurement decisions, cost structures, and sourcing strategies. Manufacturers that rely on globally dispersed component suppliers experienced higher input costs and elevated logistics complexity, which in turn affected production planning and inventory practices. Many suppliers accelerated regionalization and supplier diversification efforts to mitigate exposure to tariff volatility, while some providers adopted hedging strategies and longer-term supply agreements to stabilize procurement pricing and availability.Healthcare providers faced trade-offs between absorbing higher device-related costs or seeking alternative devices and suppliers that offered more favorable procurement terms. Capital equipment purchasers placed greater emphasis on total lifecycle costs and service agreements, seeking bundled maintenance and consumables pricing to limit year-over-year variability. The tariff changes also intensified conversations around domestic manufacturing and eligible procurement incentives, prompting several manufacturers to evaluate nearshoring, assembly localization, or strategic partnerships with regional contract manufacturers. Across the ecosystem, the net effect was a reorientation toward supply-chain transparency, contractual flexibility, and procurement practices that prioritize continuity of care and predictable operating budgets
Deconstructing the competitive and clinical contours across device type, application, end user, and operation mode to surface targeted commercialization opportunities
Segment-based insights that illuminate demand drivers, clinical utility, and competitive dynamics across device typologies, applications, end users, and operation modes
Device type segmentation reveals differentiated adoption dynamics and investment priorities. Endoscopes and visualization systems command attention due to their centrality in procedural success and their ongoing upgrade cycle, with camera systems, flexible endoscopes, and rigid endoscopes representing distinct clinical use cases and upgrade paths. Energy devices, which include electrosurgical devices, laser devices, and ultrasonic devices, are being evaluated not just for hemostasis and tissue dissection performance but for integration with visualization platforms and workflow ergonomics. Insufflators, laparoscopic instruments, morcellators, and tissue retrieval bags each present unique procurement considerations tied to safety protocols, reprocessing requirements, and single-use versus reusable economics.Application-based segmentation highlights where clinical need and innovation are most tightly coupled. Cardiovascular, ENT, general surgery, gynecology, and urology applications drive differential device design choices, training demands, and clinical support models. End user segmentation underscores operational priorities among ambulatory surgical centers, hospitals, and specialty clinics, with ambulatory settings prioritizing throughput and disposables while hospitals weigh capital investment, integration, and long-term service support. Operation mode segmentation between disposable and reusable devices further complicates procurement decisions as stakeholders evaluate infection control imperatives, environmental considerations, and lifecycle costs. Taken together, these segment lenses reveal that winning propositions must be tailored to clinical workflows, procurement frameworks, and institutional risk tolerances
Regional outlooks that map adoption maturity, regulatory nuance, and infrastructure capability across the Americas, Europe Middle East & Africa, and Asia-Pacific regions
Regional perspectives that emphasize divergent adoption patterns, regulatory influences, and infrastructure readiness across global geographies
The Americas region exhibits mature adoption pathways for advanced visualization and energy devices driven by established hospital systems and a dense network of ambulatory surgical centers. Procurement cycles in this geography often prioritize proven clinical outcomes and long-term service partnerships, and regulatory frameworks emphasize device safety and post-market surveillance. Europe, Middle East & Africa encompasses a diverse set of markets where regulatory harmonization and varying payer structures create a mosaic of adoption timelines; advanced centers in Western Europe and select Middle Eastern hubs lead in clinical sophistication, while other markets present opportunities for cost-effective, robust device designs that address infrastructure constraints.Asia-Pacific features dynamic innovation ecosystems and rapidly expanding procedural capacity, with a mix of high-volume public hospitals and private specialty centers driving demand for scalable solutions. Local manufacturing capabilities and a rising cohort of domestic device innovators are reshaping competitive dynamics, and regulatory pathways in several markets have been streamlined to encourage adoption while maintaining safety standards. Across regions, differences in reimbursement, procurement frameworks, and clinical training ecosystems necessitate region-specific go-to-market strategies that account for regulatory nuance, infrastructure readiness, and payer expectations
Competitive and strategic company-level insights that identify differentiation levers including clinical evidence, service models, and flexible manufacturing capabilities
Corporate dynamics and competitive positioning insights that matter for stakeholders evaluating partnerships, M&A, and product development priorities
The competitive landscape is shaped by a mix of established medical device corporations, specialized surgical technology firms, and emerging innovators focused on discrete segments of endosurgery. Large diversified players continue to leverage global distribution networks, comprehensive service portfolios, and integrated product suites to maintain relationships with major health systems. Niche specialists differentiate through focused clinical evidence, targeted product features, or cost-competitive manufacturing, and they often become acquisition targets when their technologies demonstrate clear clinical or operational advantages.Strategic imperatives for companies include building clinical evidence that ties device performance to patient outcomes, investing in service and training capabilities that reduce adoption friction, and ensuring manufacturing flexibility to respond to supply chain shocks. Collaboration between device makers and digital health firms is deepening, particularly in areas of imaging analytics, procedural guidance, and remote training. For stakeholders evaluating partnerships or M&A, the most compelling opportunities combine defensible intellectual property, a clear clinical value proposition, scalable manufacturing, and a pathway to integrate into health system workflows
Actionable, prioritized recommendations for manufacturers, providers, and investors to de-risk operations, accelerate clinical adoption, and enhance total cost of care outcomes
Practical, prioritized recommendations for industry leaders to accelerate adoption, mitigate risk, and capture value in a fast-evolving endosurgery environment
Manufacturers should prioritize integrations that simplify clinician workflows and reduce procedural complexity, aligning product development with the interoperability expectations of health systems and ambulatory centers. Investing in robust clinical evidence generation, including real-world evidence and outcomes-based studies, will strengthen value propositions and support procurement discussions. To reduce exposure to supply chain volatility, firms ought to diversify supplier bases, consider regional assembly or co-manufacturing arrangements, and structure service contracts that provide predictable lifecycle revenue.Providers and procurement leaders should adopt a total-cost-of-care mindset that evaluates devices across clinical outcomes, reprocessing costs, and ease of integration into existing platforms. Training programs and structured onboarding pathways reduce adoption friction and improve clinician confidence. Investors and strategic partners should prioritize companies that demonstrate clear regulatory compliance, reproducible clinical benefit, and the manufacturing agility to scale. Across the ecosystem, transparent communication, flexible contracting, and an emphasis on patient safety will be decisive in the successful adoption of next-generation endosurgery technologies
Methodological transparency outlining the expert interviews, literature review, and evidence triangulation processes behind the endosurgery device analysis
Transparent research methodology describing data sources, validation techniques, and analytic frameworks used to compile the endosurgery device insights
The analysis integrates primary qualitative input from clinicians, procurement leaders, and supply chain executives alongside proprietary device performance summaries and regulatory documentation. Expert interviews were conducted to capture frontline experiences with device usability, clinical outcomes, and procurement dynamics, enabling nuanced interpretation of how technologies are deployed across care settings. Secondary sources including peer-reviewed clinical literature, regulatory filings, and manufacturer technical specifications were systematically reviewed to corroborate thematic findings and to ensure alignment with documented device capabilities and safety profiles.Data synthesis employed triangulation across multiple lines of evidence to validate assertions and to surface consistent patterns across geographies and application areas. Where limitations existed, particularly with respect to nascent technologies and proprietary clinical datasets, findings were framed conservatively to avoid overstatement. Quality assurance procedures included cross-review by subject-matter experts, consistency checks against clinical guidelines, and verification of regulatory statuses. The methodology emphasizes transparency, reproducibility, and an evidence-weighted approach to ensure that recommendations are grounded in verifiable clinical and operational realities
A strategic synthesis highlighting the imperative to align product innovation, regulatory readiness, and operational resilience to win in the endosurgery device arena
Concluding synthesis that ties technological trends, regulatory pressures, and operational realities into a cohesive strategic outlook for endosurgery stakeholders
The endosurgery device landscape is being reshaped by technological convergence, evolving care delivery models, and shifting supply chain expectations. These forces collectively favor solutions that deliver measurable clinical benefits, seamless integration into clinical workflows, and demonstrable safety across their lifecycle. Regulatory attention and procurement practices are increasingly oriented toward long-term value and traceability, prompting manufacturers to invest in evidence generation, service excellence, and manufacturing flexibility. Providers must balance operational efficiency with clinical quality, deploying rigorous training and lifecycle management to maximize device value.Looking ahead, the most successful market participants will be those that combine product innovation with strong evidence, resilient sourcing strategies, and deep customer engagement. Strategic collaborations, targeted investments in regional manufacturing, and priority focus on interoperability and clinician-centered design will shape who leads adoption. For stakeholders across the ecosystem, the imperative is clear: align commercial strategy with clinical realities and institutional risk tolerances to translate innovation into sustained, meaningful improvements in patient care
Additional Product Information:
- Purchase of this report includes 1 year online access with quarterly updates.
- This report can be updated on request. Please contact our Customer Experience team using the Ask a Question widget on our website.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
16. China Endosurgery Device Market
Companies Mentioned
The key companies profiled in this Endosurgery Device market report include:- B. Braun Melsungen AG
- Boston Scientific Corporation
- ConMed Corporation
- Hologic, Inc.
- Intuitive Surgical, Inc.
- Johnson & Johnson
- KARL STORZ SE & Co. KG
- Medtronic plc
- Olympus Corporation
- Smith & Nephew plc
- Stryker Corporation
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 183 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
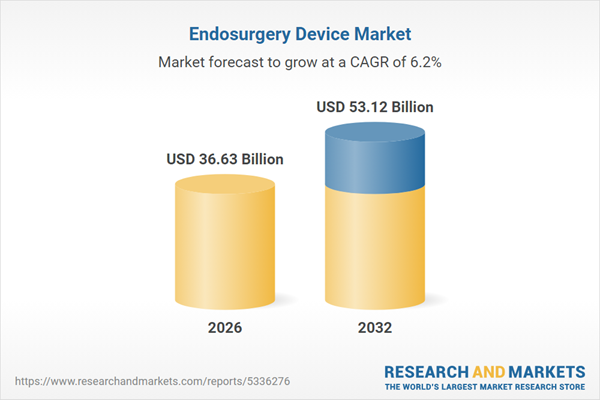
| Estimated Market Value ( USD | $ 36.63 Billion |
| Forecasted Market Value ( USD | $ 53.12 Billion |
| Compound Annual Growth Rate | 6.2% |
| Regions Covered | Global |
| No. of Companies Mentioned | 12 |