Technological developments are improving the area's capacity for discovery even more. High-throughput screening, multi-omics integration, AI-enabled target selection, and advanced antibody-engineering platforms are raising the likelihood of clinical success and enhancing early-stage efficiency. High R&D expenses, complicated biologics manufacturing, and differences in research capacity and skilled labor among European nations are some of the structural issues that the sector still faces. Nevertheless, Europe is positioned to play a major role in determining the direction of biologics drug development and next-generation therapeutic innovation due to growing investments, increased outsourcing to specialized CROs and CDMOs, and a growing shift toward personalized medicine.
Market Introduction
The Europe biologics drug discovery market is undergoing a transitional period, propelled by swift scientific advancements, changing therapeutic requirements, and robust investment activity throughout the area. Europe has become a vital center for advanced discovery platforms, antibody engineering, cell and gene therapy research, and next-generation modalities like RNA therapeutics and multispecific antibodies as biologics continue to surpass conventional small-molecule drugs in clinical success and commercial value.The region's emphasis on high-value biologics is being strengthened by aging populations, an increase in the prevalence of chronic diseases, and a growing need for tailored, targeted treatments. Collaborations between pharmaceutical corporations, biotech startups, CROs, and CDMOs are growing in order to expedite translational development, optimize lead candidates, and speed up hit identification. Early-stage research is being transformed by digital technologies, particularly AI-driven protein design, in silico modeling, and data-driven biomarker identification.
At the same time, Europe's changing regulatory landscape, especially the new EU HTA framework, is requiring developers to create more comparative and real-world evidence earlier in the pipeline. Although this makes things more complicated, it also guarantees excellent innovation and quicker adoption of ground-breaking treatments. All things considered, Europe is establishing itself as a world leader in biologics discovery by fusing scientific brilliance with an expanding network of research and commercialization partners.
Market Segmentation:
Segmentation 1: By Manufacturing Type
- In-House Manufacturing
- Outsourced Manufacturing
Segmentation 2: By Type
- Monoclonal Antibodies
- Recombinant Proteins
- Other Biologics
Segmentation 3: By Region
- Europe
- Germany
- U.K.
- France
- Italy
- Spain
- Rest-of-Europe
Europe Biologics Drug Discovery Market Trends, Drivers and Challenges
Market Trends
- Strong expansion of biologics as one of Europe’s fastest-growing pharmaceutical segments.
- Shift toward advanced modalities such as cell and gene therapies, RNA therapeutics, multispecifics, and ADCs.
- Rising integration of AI, machine learning, and computational protein design to accelerate early-stage discovery.
- Increased partnerships, licensing deals, and outsourcing to CROs/CDMOs for specialized discovery capabilities.
- Growing biosimilar development influencing portfolio decisions and lifecycle management.
Market Growth Drivers
- Advancements in high-throughput screening, single-cell analysis, and structural biology speeding up target validation.
- Ageing population and rising prevalence of chronic and rare diseases boosting demand for innovative biologics.
- Strong investment inflow from pharma, biotech, VC, and strategic collaborations supporting R&D.
- Supportive innovation initiatives and evolving regulatory frameworks encouraging advanced biologics.
- Increasing use of real-world data and digital platforms for biomarker research and target selection.
Market Challenges
- Complex and fragmented regulatory and HTA pathways across Europe affecting approval and access timelines.
- Intensifying pricing pressure and reimbursement hurdles due to payer scrutiny and biosimilar competition.
- High manufacturing and CMC complexity leading to elevated development costs and longer timelines.
- Talent shortage in specialized areas like protein engineering, biologics CMC, and regulatory sciences.
- Supply-chain vulnerabilities linked to dependence on external regions for APIs and CDMO capacity.
How can this report add value to an organization?
Product/Innovation Strategy: The Europe biologics drug discovery market has been extensively segmented based on various categories, such as manufacturing type, type, and region. This can help readers get a clear overview of which segments account for the largest share and which ones are well-positioned to grow in the coming years.Growth/Marketing Strategy: Partnerships, alliances, and business expansions have accounted for the majority of key developments.
Competitive Strategy: The Europe biologics drug discovery market has numerous established players with product portfolios. Key players in the Europe biologics drug discovery market, analyzed and profiled in the study, include established players offering platforms, products, and services for biologics drug discovery.
Key Market Players and Competition Synopsis
The companies profiled have been selected based on inputs gathered from an analysis of company coverage, product portfolio, and market penetration.Some prominent names established in this market are:
- AstraZeneca Plc
- Evotec SE
- Merck KGaA
- Novo Nordisk A/S
- Novartis AG
- F. Hoffmann-La Roche AG
This product will be delivered within 2 business days.
Table of Contents
Companies Mentioned
- AstraZeneca Plc
- Evotec SE
- Merck KGaA
- Novo Nordisk A/S
- Novartis AG
- F. Hoffmann-La Roche AG
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 81 |
| Published | December 2025 |
| Forecast Period | 2025 - 2035 |
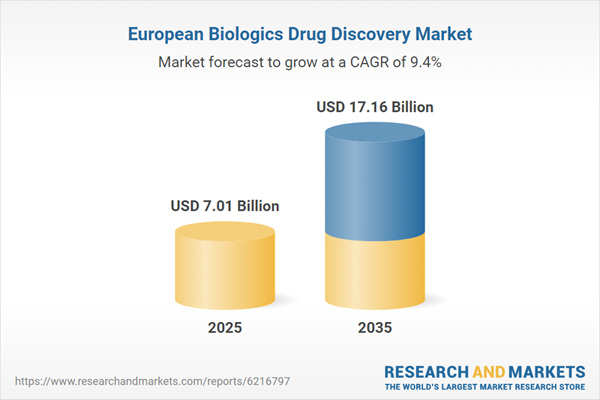
| Estimated Market Value ( USD | $ 7.01 Billion |
| Forecasted Market Value ( USD | $ 17.16 Billion |
| Compound Annual Growth Rate | 9.3% |
| Regions Covered | Europe |
| No. of Companies Mentioned | 6 |