Speak directly to the analyst to clarify any post sales queries you may have.
Comprehensive introduction to expression vectors explaining their central role in enabling recombinant biology, therapeutics development, and translational workflows
Expression vectors are foundational tools that enable recombinant protein expression, functional genomics, and gene-based therapeutics across industrial, academic, and clinical domains. These biological platforms underpin a range of activities from early discovery assays and high-throughput screening to advanced gene therapy product development and vaccine manufacture. The diversity of vector architectures, host systems, and expression strategies creates a layered ecosystem in which scientific innovation, manufacturing capabilities, and regulatory pathways interact in increasingly complex ways.Because expression vectors sit at the intersection of molecular biology and biomanufacturing, their role extends beyond reagent supply to shaping timelines, costs, and feasibility of translational projects. Advances in vector design, delivery modalities, and host optimization have narrowed the gap between bench-scale proof-of-concept and clinical-grade production, but they also demand rigorous quality control, scalable analytics, and adaptable supply chains. In this context, stakeholders must evaluate vectors not only on immediate performance metrics but also on compatibility with downstream processes, regulatory expectations, and commercial manufacturing constraints.
Looking forward, the trajectory of expression vector utility is influenced by broader technological trends such as modular design principles, platform standardization, and increased emphasis on reproducibility. These drivers are reshaping procurement, R&D priorities, and collaboration models across academia, contract service providers, and industry, making a clear-eyed understanding of vector capabilities and limitations essential for effective strategic planning.
Transformative shifts reshaping the expression vectors landscape driven by engineering advances, manufacturing modernization, and regulatory harmonization
The landscape of expression vectors is undergoing transformative shifts driven by converging technological, regulatory, and commercial forces. Advances in vector engineering-such as precision capsid modification for viral vectors and optimized regulatory elements for plasmid systems-have substantially improved transduction efficiency and expression stability, enabling applications that were previously infeasible. Concurrently, innovations in host engineering, including refined CHO and HEK293 expression platforms and next-generation yeast strains, are increasing yields and product quality while reducing development timelines.Manufacturing paradigms are also evolving. Single-use technologies, intensified processes, and platform-based upstream and downstream workflows are lowering barriers to scalable production of both viral and non-viral vectors. This operational maturation has been accelerated by cross-sector collaborations and investments in specialized contract manufacturing organizations that offer end-to-end capabilities. At the same time, digitalization and advanced analytics are bringing higher-resolution process monitoring and predictive quality control, facilitating faster process transfers and improved reproducibility.
From a regulatory perspective, agencies are progressively clarifying expectations around characterization, potency assays, and comparability, which is encouraging standardized approaches and supporting broader clinical adoption. Taken together, these shifts are enabling a transition from small-batch, bespoke vector development to more standardized, industrialized processes that can meet the demands of advanced therapeutics and high-throughput research applications.
Assessment of how tariff changes and trade policy shifts are reshaping supply chains, sourcing strategies, and manufacturing localization for expression vectors
Policy actions affecting tariffs and trade can materially influence supply chains for biological reagents, equipment, and contract services that support expression vector development and manufacturing. Tariff adjustments often raise direct input costs for critical raw materials such as plasmid DNA production reagents, single-use consumables, chromatography media, and specialized enzymes, which can compress margins for suppliers and increase procurement costs for research organizations and manufacturers. These cost pressures tend to reverberate through pricing negotiations, supplier selection, and outsourcing strategies.Beyond direct cost implications, tariff-driven disruptions can redirect sourcing strategies and accelerate localization of manufacturing capacity. Organizations dependent on cross-border supply relationships may pursue greater vertical integration or shift to regional suppliers to mitigate exposure to trade volatility. This reorientation increases the strategic value of domestic contract development and manufacturing organizations and stimulates investment in local infrastructure, but it may also lengthen lead times for certain specialized components that are still concentrated in limited global hubs.
Tariffs can also affect collaborative research models, international partnerships, and clinical supply chains by altering the economics of sample and material transfers. Regulatory compliance and customs processes may become more complex, requiring enhanced documentation and forward-planning to avoid delays in clinical material shipments. In aggregate, these dynamics incentivize a robust supply chain risk management approach that combines supplier diversification, inventory optimization, and strategic investments in capacity expansion to sustain R&D momentum and protect product development timelines.
In-depth segmentation insights revealing how vector architectures, host systems, expression strategies, applications, and end-user types define distinct operational and commercial requirements
A coherent segmentation framework clarifies how different vector types, host systems, expression strategies, applications, and end users each create distinct value chains and technical requirements. Based on vector type, practitioners evaluate Bacterial Artificial Chromosome constructs, plasmid vectors, viral systems, and Yeast Artificial Chromosome solutions, and within viral systems attention focuses on adeno-associated virus (AAV) serotypes, adenoviral platforms, lentiviral backbones, and retroviral vectors, each of which presents unique potency, tropism, and manufacturing considerations. Based on host organism, choices span bacterial, insect, mammalian, and yeast systems, with mammalian hosts often narrowed to CHO cells and HEK293 lines when higher-order post-translational modifications are required, and yeast hosts commonly represented by Pichia pastoris and Saccharomyces cerevisiae for cost-effective expression of certain protein classes.Based on expression system, there is a practical distinction between stable expression approaches and transient expression strategies; stable platforms are frequently implemented using antibiotic selection or metabolic marker systems to maintain long-term expression, while transient methods rely on techniques such as electroporation, lipofection, or viral transduction to achieve rapid, short-term protein production. Based on application, vectors are deployed across diagnostics, research, and therapeutics, where diagnostics may emphasize imaging and molecular diagnostic reagents, research encompasses basic discovery and drug discovery workflows, and therapeutics includes gene therapy, protein replacement strategies, and vaccine development. Based on end user, the ecosystem is composed of academic and research institutes-with government labs and universities playing major roles-contract research organizations that provide development services, and pharmaceutical and biotech companies that range from nimble biotech firms to large pharmaceutical organizations, each with differing procurement, quality, and scalability expectations.
Understanding these segmentation dimensions enables more targeted product development, commercialization strategies, and service offerings by aligning technical capabilities with end-user needs and regulatory requirements.
Key regional insights highlighting distinct strengths in research ecosystems, manufacturing capabilities, and regulatory frameworks across major global regions
Regional dynamics exert a strong influence on research priorities, manufacturing capacity, regulatory alignment, and talent availability across the global expression vectors ecosystem. In the Americas, the interplay between leading academic centers, a mature biotech industry, and a robust contract services sector supports rapid translation of vector technologies into clinical candidates and commercial products. This region’s strength in venture funding and entrepreneurial ecosystems accelerates early-stage innovation and fosters close partnerships between research institutions and industry.Europe, Middle East & Africa blends advanced biomanufacturing hubs, stringent regulatory frameworks, and strong academic-industry linkages that encourage high compliance standards and process robustness. Regulatory harmonization efforts and collaborative initiatives within Europe promote the sharing of best practices for vector characterization and quality assurance, which benefits multinational development programs and contract manufacturing relationships. In contrast, the Asia-Pacific region is notable for rapid capacity expansion, competitive manufacturing costs, and growing technical expertise across host systems and process intensification. Investments in local infrastructure and a rising number of clinical programs have positioned Asia-Pacific as a critical source of both reagents and specialized manufacturing services, while diverse regulatory environments create both opportunities and challenges for cross-border development.
These regional characteristics underscore the need for differentiated commercial strategies, supply chain configurations, and regulatory engagement plans that reflect local strengths in R&D, manufacturing, and policy development.
Key company-level insights showing how specialized suppliers, CMOs, and platform providers are driving innovation through partnerships, scale, and analytical capabilities
The competitive landscape in expression vectors is characterized by a mix of specialty reagent suppliers, repository services, contract manufacturing organizations, and vertically integrated life science companies. Specialty suppliers focus on high-quality plasmid production, enzyme systems, and single-use consumables that are essential to both small-scale research and large-scale manufacturing. Repository and distribution services play an outsized role in enabling reproducibility and rapid access to characterized vector backbones and plasmids, while contract manufacturers provide critical scale-up, cGMP production, and fill-finish capabilities for both viral and non-viral vectors.Across the ecosystem, there is a clear trend toward partnerships and strategic alliances that combine design expertise with manufacturing capacity and regulatory know-how. Innovation is frequently concentrated around improved vector design for enhanced potency and safety, advances in analytics and release testing, and platformization that reduces time-to-clinic for successive programs. Companies that invest in robust quality systems, scalable process development, and advanced analytics are increasingly favored by large developers seeking reliable supply and streamlined regulatory submissions. At the same time, emerging players often differentiate through niche specialization-such as bespoke AAV serotype engineering, plasmid backbone optimization, or novel host strain development-creating opportunities for targeted collaboration and acquisition within the sector.
Actionable recommendations for leaders to strengthen supply resilience, standardize platform approaches, and accelerate regulatory alignment for expression vector programs
Industry leaders should adopt a strategic agenda that balances near-term risk mitigation with longer-term capability building. First, prioritize diversification of critical suppliers and consider dual sourcing for high-risk inputs to reduce exposure to trade policy shifts and single-point failures. Simultaneously, evaluate selective investments in regional manufacturing capacity or partnerships with established contract manufacturers to shorten supply chains, accelerate clinical supply availability, and support regulatory filing strategies.Second, accelerate adoption of platform approaches that standardize vector backbones, expression elements, and analytics to reduce development variability and enable faster technology transfer. Invest in advanced process analytics and automation to improve reproducibility, reduce waste, and support rapid scale-up. Third, engage proactively with regulatory authorities to align on characterization requirements, potency assays, and comparability strategies; early dialogue can streamline review pathways and minimize costly late-stage changes.
Fourth, strengthen talent pipelines by investing in workforce development for cell line engineering, process development, and quality sciences, and cultivate collaborations with academic centers to access cutting-edge research. Fifth, incorporate sustainability and supply chain transparency into procurement and operational planning to meet stakeholder expectations and regulatory scrutiny. Finally, adopt flexible commercialization models that include licensing, strategic partnerships, and bespoke service offerings to capture value across the research-to-therapy continuum.
Transparent research methodology describing primary interviews, secondary evidence synthesis, triangulation, and validation steps used to derive strategic insights
The research methodology underpinning this executive summary integrates multiple sources and validation steps to ensure analytical rigor and practical relevance. Primary research included structured interviews with technical leaders across academic institutions, contract development and manufacturing organizations, and in-house R&D teams from industry, supplemented by detailed technical reviews of peer-reviewed literature and regulatory guidance documents to ground observations in current scientific and policy frameworks. Secondary research comprised systematic synthesis of publicly available technical publications, patents, and conference proceedings to capture recent innovations in vector engineering, host optimization, and manufacturing process design.Triangulation of findings was achieved through cross-validation of interview insights with documented studies and process case examples, ensuring that reported trends reflect both experiential and evidentiary bases. Segmentation mapping was used to align technical attributes with application demands and end-user needs, and sensitivity checks were applied to assess how supply chain or policy changes could affect different parts of the ecosystem. The methodology also considered limitations, including variability in proprietary process details and the evolving nature of clinical regulatory guidance, which were addressed by focusing on functional impacts and strategic implications rather than proprietary performance metrics.
Ethical considerations and data integrity were maintained throughout by using anonymized interview data where requested and relying on verified, citable technical sources for scientific claims. This blended approach ensures that the conclusions and recommendations are actionable, defensible, and relevant to decision-makers.
Strategic conclusion summarizing how technological progress, regulatory clarity, and supply resilience collectively determine success in the expression vectors ecosystem
The synthesis presented here underscores the centrality of expression vectors to modern biological innovation and highlights how technological advances, regional dynamics, and supply chain considerations collectively shape strategic choices for stakeholders. Vector design improvements, host optimization, and manufacturing modernization are enabling broader therapeutic and diagnostic applications, while regulatory clarity and platform standardization are facilitating more predictable development pathways. At the same time, external pressures such as trade policy shifts and competitive regional investments require purposeful supply chain planning and targeted capacity development.For organizations operating in this space, success hinges on aligning technical decisions with commercial and regulatory realities, investing in scalable analytics and process robustness, and forging partnerships that combine scientific creativity with manufacturing excellence. By emphasizing resilience, standardization, and strategic collaboration, stakeholders can navigate an increasingly complex landscape and translate vector innovations into reliable research tools and viable therapeutic products. The collective momentum across academic, service provider, and industry sectors points to a maturing ecosystem that is capable of supporting both high-throughput discovery and the rigorous demands of clinical development.
Additional Product Information:
- Purchase of this report includes 1 year online access with quarterly updates.
- This report can be updated on request. Please contact our Customer Experience team using the Ask a Question widget on our website.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
17. China Expression Vectors Market
Companies Mentioned
- Addgene, Inc.
- Agilent Technologies, Inc.
- GenScript Biotech Corporation
- Lonza Group AG
- Merck KGaA
- New England Biolabs, Inc.
- OriGene Technologies, Inc.
- Promega Corporation
- Takara Bio Inc.
- Thermo Fisher Scientific Inc.
- VectorBuilder, Inc.
- WuXi Biologics
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 198 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
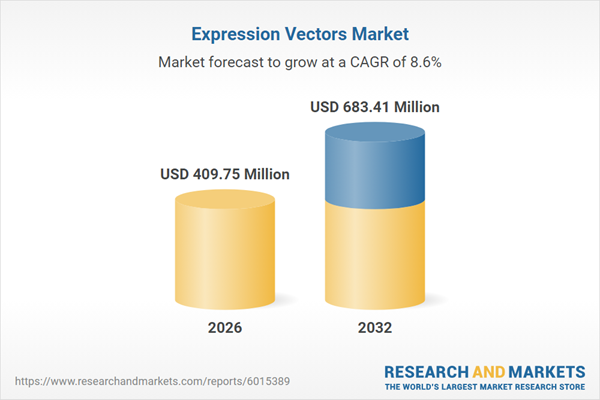
| Estimated Market Value ( USD | $ 409.75 Million |
| Forecasted Market Value ( USD | $ 683.41 Million |
| Compound Annual Growth Rate | 8.5% |
| Regions Covered | Global |
| No. of Companies Mentioned | 12 |