Smallpox Treatment:
Key Trends and DriversThe medical management of smallpox, a severe infectious disease declared eradicated in 1980, largely focuses on supportive care as no definitive treatment exists. With concerns about its potential use as a bioweapon, the treatment protocol involves isolation of infected patients until all scabs have fallen off to prevent virus transmission. With the FDA's approval of drugs like tecovirimat in 2018 and brincidofovir in 2021, there has been significant advancement in the therapeutic options available, demonstrating effectiveness in animal studies and approved under protocols for emergency use. The FDA plays a critical role in ensuring the availability of medical countermeasures against smallpox through facilitating drug development and ensuring the safety of the nation's blood supply. Vaccines like ACAM2000, Jynneos, and the investigational Aventis Pasteur Smallpox Vaccine are crucial in the Strategic National Stockpile, ready for deployment in case of an outbreak. Treatment drugs such as tecovirimat, available in both oral and intravenous forms, and brincidofovir are part of the emergency resources.
Several factors drive the adoption of smallpox treatments and preventive measures despite the eradication of the disease decades ago. Primarily, the persistent threat of bioterrorism where smallpox could be used as a biological weapon significantly influences government policies and healthcare strategies worldwide. This potential threat ensures continued research into and stockpiling of vaccines and antiviral medications. Technological advances in medical research allow for more effective and safer smallpox vaccines and treatments, increasing their adoption. Furthermore, global health security initiatives emphasize the importance of preparedness for potential outbreaks, pushing for advancements in diagnostic and therapeutic options. Public health policies also play a crucial role; they mandate certain protocols for handling outbreaks, which include the maintenance of strategic reserves of vaccines and antivirals. Finally, international cooperation and funding for infectious disease control further support the readiness and availability of smallpox medical countermeasures, reinforcing a global shield against possible future outbreaks.
Report Scope
The report analyzes the Smallpox Treatment market, presented in terms of market value (US$ Thousand). The analysis covers the key segments and geographic regions outlined below.- Segments: Treatment Type (Antiviral Drugs, Vaccinations, Supportive Care); End-Use (Hospitals & Clinics End-Use, Other End-Uses).
- Geographic Regions/Countries:World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Antiviral Drugs segment, which is expected to reach US$32.5 Million by 2030 with a CAGR of a 1.6%. The Vaccinations segment is also set to grow at 2% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $18.1 Million in 2024, and China, forecasted to grow at an impressive 3% CAGR to reach $13.5 Million by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Smallpox Treatment Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Smallpox Treatment Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Smallpox Treatment Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Bavarian Nordic AS, Chimerix, Inc., Emergent BioSolutions Inc., Emergex Vaccines, EpiVax, Inc. and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 86 companies featured in this Smallpox Treatment market report include:
- Bavarian Nordic AS
- Chimerix, Inc.
- Emergent BioSolutions Inc.
- Emergex Vaccines
- EpiVax, Inc.
- Oncovir, Inc.
- Pfizer, Inc.
- Sanofi SA
- SIGA Technologies Inc
- Tonix Pharmaceuticals Holding Corp.
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Bavarian Nordic AS
- Chimerix, Inc.
- Emergent BioSolutions Inc.
- Emergex Vaccines
- EpiVax, Inc.
- Oncovir, Inc.
- Pfizer, Inc.
- Sanofi SA
- SIGA Technologies Inc
- Tonix Pharmaceuticals Holding Corp.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 320 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
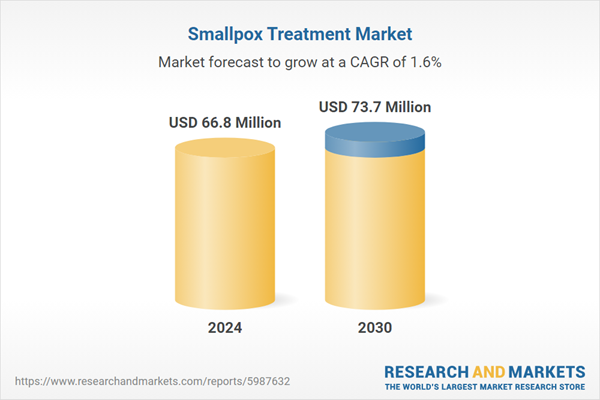
| Estimated Market Value ( USD | $ 66.8 Million |
| Forecasted Market Value ( USD | $ 73.7 Million |
| Compound Annual Growth Rate | 1.6% |
| Regions Covered | Global |