Speak directly to the analyst to clarify any post sales queries you may have.
An authoritative framing of spinal implant dynamics that synthesizes clinical innovation, delivery model shifts, and strategic imperatives shaping industry decision-making
The spinal implants sector sits at the confluence of surgical innovation, demographic pressure, and evolving clinical practice, creating a high-stakes environment for device developers, health systems, and investors. Advances in materials science, biomechanics, and imaging-guided implantation have altered clinical expectations, while shifts in care delivery models and payer scrutiny are reshaping value propositions. Consequently, stakeholders must navigate tighter regulatory oversight, growing demand for minimally invasive procedures, and a pronounced emphasis on long-term patient outcomes. In this context, a clear, concise framing of market dynamics is essential for leaders who must balance investment in novel technologies with proven clinical performance.Over recent years, the landscape has seen a pronounced acceleration in technologies designed to reduce operative time, improve anatomical restoration, and enhance fusion rates or motion preservation where clinically appropriate. Concurrently, hospitals and ambulatory surgical centers are optimizing care pathways to shorten length of stay and improve throughput, which places a premium on implants that streamline procedures and minimize complications. As a result, decision-makers are increasingly evaluating innovations not merely on clinical merit but on total cost of care, device lifecycle management, and compatibility with evolving reimbursement frameworks. This introduction sets the stage for a deeper exploration of shifting paradigms, tariff implications, segmentation intelligence, regional distinctions, competitive positioning, and practical recommendations that support informed strategic decisions.
How converging technological advances, procedural migration to outpatient care, and heightened evidence expectations are reshaping product development and commercialization strategies
Fundamental shifts are underway across the spinal implants landscape, driven by converging forces that redefine product development priorities and commercialization strategies. Technological progress in interbody device geometry, additive manufacturing, and surface engineering is enabling implants to better mimic native biomechanics, thereby expanding clinical indications and surgeon acceptance. At the same time, there is a clear move toward motion-preserving solutions and modular systems that allow intraoperative flexibility, which responds to both surgeon preference and patient-specific anatomical variability. These technical developments are accompanied by a growing emphasis on data-driven device performance, where registries, real-world evidence, and post-market surveillance increasingly inform adoption curves and payer conversations.Simultaneously, the ecosystem for care delivery is transforming. Ambulatory surgical centers are capturing procedures that historically required inpatient settings, creating demand for implants that support minimally invasive approaches and rapid recovery. Supply chain resilience and procurement strategies have also changed; hospitals and group purchasing organizations are prioritizing interoperability, vendor consolidation, and total-cost analyses. Regulatory pathways have evolved to place greater scrutiny on clinical claims and long-term safety, prompting manufacturers to invest earlier in robust clinical programs. Taken together, these transformative shifts are reshaping how new entrants and incumbents allocate R&D capital, structure clinical evidence generation, and position product portfolios for sustained adoption.
Anticipating the strategic ripple effects of tariff-driven cost pressures on supply chain design, sourcing decisions, and competitive dynamics through 2025
Policy actions affecting cross-border trade and tariffs have ripple effects across complex medical device supply chains, and the cumulative influence of tariff activity through 2025 will be mediated by specific policy choices and industry responses. Historically, tariffs on steel and aluminum and periodic trade actions have introduced raw material cost volatility that is particularly relevant for spinal implants manufactured from titanium, stainless steel, and composite-metal constructs. If tariff measures target finished medical components or intermediate inputs, manufacturers face choices between absorbing cost pressure, passing costs to customers, or altering supply chain footprints by reshoring or nearshoring production. Each approach carries operational trade-offs: absorbing costs compresses margins, passing costs strains procurement budgets, and re-siting production requires capital investment and regulatory revalidation.Moreover, tariffs compound existing pressures stemming from logistic constraints and elevated supplier risk, thereby incentivizing strategic repositioning. In practice, manufacturers may diversify supplier bases to mitigate exposure, increase vertical integration for critical components, or pursue more localized partnerships to reduce lead time and tariff exposure. Transitional strategies often include contract renegotiations with health systems and value-based pricing initiatives that emphasize demonstrable outcomes. In addition, tariff-driven cost shifts can accelerate consolidation as smaller manufacturers find it harder to sustain competitive pricing, while larger firms leverage scale and integrated manufacturing to absorb disruptions. Ultimately, the cumulative impact of tariff dynamics through 2025 is best viewed as a catalyst for supply chain optimization, strategic sourcing diversification, and heightened emphasis on cost transparency across procurement and clinical decision-making.
Deep segmentation intelligence that reconciles product taxonomy, clinical applications, end user pathways, material science choices, and sales channel dynamics to reveal strategic focus areas
A granular understanding of segmentation is essential to align product development and commercialization strategies with clinical needs and reimbursement realities, and the market is partitioned across multiple layers that affect clinical utility and commercial pathways. Based on product type, the landscape includes Complementary Devices, Non Fusion Devices, and Spinal Fusion Devices. Complementary Devices encompass Bone Graft Substitutes, Orthobiologics, and Surgical Instruments, which play critical supporting roles in optimizing fusion biology and procedural efficiency. Non Fusion Devices include Disc Replacement Devices, Dynamic Stabilization Devices, and Growth Modulation Systems, addressing indications where motion preservation or growth guidance is preferred. Spinal Fusion Devices comprise Anterior Fusion Devices, Interbody Fusion Devices, and Posterior Fusion Devices; within anterior fusion, differentiations are seen between Interbody Cages and Vertebrectomy Implants, while interbody fusion demonstrates procedural distinctions such as Anterior Lumbar Interbody Fusion, Extreme Lateral Interbody Fusion, Lateral Lumbar Interbody Fusion, Posterior Lumbar Interbody Fusion, and Transforaminal Lumbar Interbody Fusion. Posterior fusion solutions are further categorized into Pedicle Screw Systems, Plate Systems, and Rod Systems, each with unique surgical workflows and instrumentation demands.Additionally, segmentation by application distinguishes clinical targets such as Degenerative Disc Disease, Scoliosis and Deformity, Spinal Stenosis, Trauma and Fracture, and Tumor, where therapeutic objectives and regulatory evidence expectations vary significantly. End user segmentation highlights Ambulatory Surgical Centers, Hospitals, and Specialty Clinics, and each channel presents different purchasing behaviors, procedural volumes, and value thresholds. Implant material segmentation spans Composite, Polyetheretherketone, Stainless Steel, and Titanium and Alloys, reflecting trade-offs among imaging compatibility, biomechanical properties, and manufacturing economics. Finally, sales channels typically split into Direct Sales and Distributor Sales, influencing commercial reach, service models, and pricing transparency. Synthesizing these layers clarifies where clinical unmet needs intersect with commercial opportunity and where targeted innovation or strategic partnerships can achieve differentiated impact.
Regional strategic distinctions and market access considerations that determine prioritization of clinical programs, distribution models, and localization investments to maximize adoption
Regional dynamics materially influence adoption patterns, regulatory interactions, and commercialization strategies, and recognizing these distinctions enables more effective market entry and scale plans. In the Americas, robust procedural volumes, well-established reimbursement frameworks, and a mature healthcare infrastructure create an environment where clinical evidence and cost-effectiveness increasingly drive purchasing decisions. European, Middle Eastern, and African markets display heterogeneity: Western Europe often leads in early adoption of high-cost technologies contingent on rigorous health technology assessments, while specific regulators and payers in the Middle East and Africa prioritize cost containment and scalable solutions. Asia-Pacific markets are diverse, with advanced markets demonstrating rapid uptake of minimally invasive and navigation-enabled systems, and emerging markets exhibiting strong growth potential tied to expanding surgical capacity and rising incidence of degenerative conditions.These regional differences necessitate tailored strategies: product registries and post-market surveillance are particularly influential in markets with stringent assessment protocols, while flexible pricing and tiered product lines often work better in price-sensitive regions. Channel strategies should account for the prevalence of distributor networks versus direct sales models and the maturation level of ambulatory care across regions. Furthermore, regulatory timelines and import controls vary, so manufacturing footprint decisions and regulatory dossiers must be aligned to regional requirements. Ultimately, regional insights should inform prioritization of clinical programs, distribution partnerships, and localization investments to ensure effective market penetration and sustainable growth.
Competitive positioning insights that explain how portfolio integration, evidence generation, and service models drive adoption and differentiate manufacturers in the spinal implant sector
Competitive positioning in the spinal implants arena reflects a mix of scale-based advantages, portfolio breadth, and differentiated clinical evidence, and leaders demonstrate distinct approaches to product innovation and commercial execution. Large medtech firms often deploy integrated portfolios spanning spinal fusion systems, biologics, and surgical navigation to offer bundled solutions that align with hospital value initiatives. Mid-sized and specialized companies frequently focus on niche clinical innovations, such as advanced interbody surface technologies, modular cushioning for motion-preserving devices, or targeted orthobiologic platforms that address specific indications. Across the competitive set, success commonly derives from an ability to marry rigorous clinical data with surgeon-centric training programs and scalable service models that reduce time-to-adoption.In addition, strategic partnerships, licensing deals, and selective acquisitions are frequently used to accelerate time to market and augment evidence generation. Companies investing in digital adjuncts-such as intraoperative guidance, outcomes tracking, and predictive analytics-create additional differentiation by demonstrating how devices perform in real-world settings. Procurement and hospital groups increasingly favor suppliers that provide comprehensive support across the product lifecycle, including instrument management, training, and consignment models. Finally, supply chain robustness and manufacturing flexibility remain competitive differentiators, as firms that can reliably meet demand and adapt to material availability maintain stronger relationships with large health systems and group purchasing organizations.
Actionable strategic guidance for executives to align clinical evidence, modular product design, and resilient sourcing to accelerate adoption and mitigate market risks
Industry leaders should adopt a multi-pronged approach that aligns innovation, commercial execution, and operational resilience to capture sustainable value in the spinal implants domain. Prioritize investment in clinical programs that generate high-quality, indication-specific evidence demonstrating improved outcomes and total cost of care, while integrating real-world data capture to support iterative product improvement and payer engagement. Simultaneously, design product platforms for modularity and procedural flexibility, enabling the same core components to address multiple surgical approaches and thereby reducing inventory complexity for hospital partners. To address cost volatility and policy risk, diversify sourcing strategies and evaluate nearshoring or capacity partnerships for critical components while maintaining compliance and manufacturing traceability.From a commercial perspective, tailor go-to-market models to regional dynamics and end user preferences, balancing direct sales where clinical engagement is paramount with distributor partnerships in price-sensitive or geographically fragmented markets. Strengthen value-based contracting capabilities and supplier transparency to align with health system procurement priorities. Invest in surgeon education and simulation-based training programs to accelerate adoption and reduce learning curves, and offer outcome-linked service packages that bundle instrumentation, training, and data analytics. Finally, embed sustainability and cybersecurity considerations into product design and post-market surveillance to meet evolving regulatory expectations and institutional procurement criteria, thereby reducing friction during tender processes and facilitating long-term partnerships.
A transparent, multi-source research methodology that integrates clinician interviews, regulatory and clinical literature review, and supply chain analysis to validate strategic insights
This research synthesizes primary interviews with clinical and commercial stakeholders, secondary review of regulatory filings and peer-reviewed literature, and structured analysis of supply chain and policy trends to create a robust evidence base for strategic decision-making. Primary inputs include conversations with spine surgeons, hospital procurement leaders, and device developers to validate clinical utility, adoption barriers, and commercial dynamics. Secondary sources encompass regulatory guidance documents, device registries, surgical society publications, and technical literature that inform safety and efficacy considerations. Analytical steps included mapping product taxonomies against clinical workflows, evaluating material and manufacturing trade-offs, and assessing regional regulatory and reimbursement frameworks for market access implications.Data quality and triangulation were ensured through cross-validation of interview insights with publicly available clinical outcomes and manufacturer technical documentation. Where possible, longitudinal trends were corroborated with procedure volume data and device approval timelines. Limitations include variable availability of proprietary pricing arrangements and the inherent lag between clinical practice shifts and published evidence, which were mitigated by sourcing contemporaneous expert input. Overall, the methodology emphasizes reproducibility, transparency in source attribution, and practical relevance for commercial planning and regulatory strategy.
A concise synthesis of strategic priorities showing how evidence-driven innovation, supply resilience, and tailored market access create durable competitive advantage in spinal implants
In conclusion, the spinal implants sector presents a dynamic intersection of material science advances, shifting care delivery models, and intensifying evidence requirements that collectively shape strategic imperatives for stakeholders. Technological innovations in implant design and manufacturing open avenues for improved patient outcomes and procedural efficiency, yet adoption is contingent on rigorous clinical validation, cost-effectiveness, and seamless integration into evolving surgical workflows. Policy and tariff dynamics amplify the need for resilient supply chains and flexible sourcing, while regional differences necessitate tailored market access and distribution strategies. Competitively, firms that combine strong clinical evidence with scalable service models and reliable supply will be best positioned to secure sustainable partnerships with health systems and ambulatory providers.Looking forward, the most successful strategies will be those that bridge clinical excellence with operational agility: investing in targeted clinical programs, adopting modular product architectures, and aligning commercialization models with payer and hospital priorities. By focusing on demonstrable outcomes, supplier transparency, and regional adaptability, industry leaders can navigate the complexities of the current environment and create differentiated value propositions that meet the needs of surgeons, patients, and payers alike.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
17. China Spinal Implants Market
Companies Mentioned
- Alphatec Holdings, Inc.
- B. Braun Melsungen AG
- Exactech, Inc.
- Globus Medical, Inc.
- Johnson & Johnson
- Medtronic plc
- NuVasive, Inc.
- Orthofix Medical Inc.
- RTI Surgical, Inc.
- SeaSpine Holdings Corporation
- Stryker Corporation
- Zimmer Biomet Holdings, Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 190 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
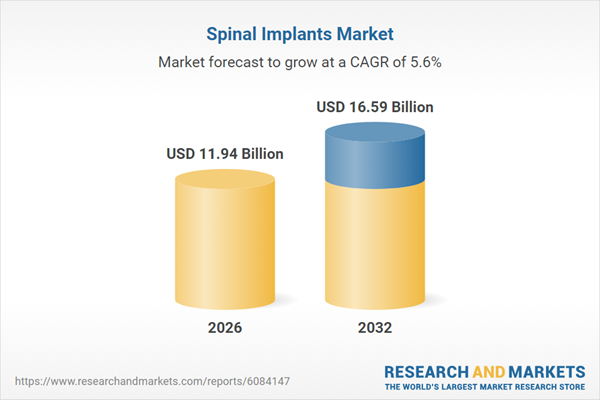
| Estimated Market Value ( USD | $ 11.94 Billion |
| Forecasted Market Value ( USD | $ 16.59 Billion |
| Compound Annual Growth Rate | 5.6% |
| Regions Covered | Global |
| No. of Companies Mentioned | 12 |