Speak directly to the analyst to clarify any post sales queries you may have.
An authoritative overview of long read sequencing evolution highlighting technological, operational, and organizational dynamics shaping research and clinical adoption
Long read sequencing has matured from an experimental niche into a strategic platform driving deeper biological insight and translational applications across research, clinical, agricultural, and industrial domains. As read lengths, raw accuracy, and throughput continue to improve, long read technologies are enabling the resolution of complex genomic regions, structural variants, and epigenetic modifications in ways that short read approaches cannot reliably deliver. This evolution has catalyzed new use cases and workflows that extend beyond pure discovery science into diagnostics development, regulatory submissions, and agrigenomics programs.Across laboratories, the integration of long read platforms has prompted reassessment of sample preparation, data analysis, and procurement strategies. Researchers are adapting wet-lab protocols to preserve long-fragment integrity while bioinformatics teams are building or adopting pipelines capable of handling larger contiguous reads and richer signal-level data. The shift is not only technological but organizational; cross-functional teams spanning molecular biology, computational biology, and regulatory affairs are converging to translate long read outputs into validated insights.
Importantly, the commercial ecosystem supporting long read sequencing has broadened to include a more diverse array of consumables, instruments, and software and services. This diversity has fostered competition and innovation, with new entrants challenging established players on price, flexibility, and application-specific performance. The result is a more dynamic landscape where end users must weigh trade-offs among instrument ecosystems, reagent availability, data processing requirements, and service-level agreements. In this context, strategic procurement, partnerships, and investment in in-house capabilities determine whether organizations capture the promised gains in resolution, reproducibility, and clinical utility.
This report unpacks these shifts by examining technology trajectories, segmentation drivers, regional dynamics, and the implications of evolving trade policies. By connecting operational realities in the laboratory with macro-level market signals, it aims to provide an actionable framework for R&D leaders, procurement officers, and corporate strategists seeking to position their organizations for sustainable adoption of long read sequencing.
Critical industry inflection points revealing how quality gains, computational advances, and ecosystem partnerships are reshaping adoption and application strategies
The long read sequencing landscape has undergone several transformative shifts that together redefine what is technically possible and commercially viable. First, improvements in raw read quality and error-correction strategies have narrowed the gap between long read and short read confidence for many variant classes, enabling broader use in diagnostic and regulatory contexts. This progression has been accompanied by a proliferation of library preparation chemistries and workflow optimizations that reduce hands-on time while increasing the proportion of high-quality long fragments recovered from challenging sample types.Second, computational innovation has matured from bespoke academic tools to robust, production-ready pipelines and commercial software suites. Enhanced basecalling, alignment, and variant-calling algorithms that are specifically tuned to long read error profiles now allow more consistent interpretation across instrument platforms. As a result, bioinformatics has become a pivotal differentiator for vendors and service providers, and for end users selecting solutions that align with their scale and regulatory aspirations.
Third, there has been a strategic realignment in partnerships and go-to-market approaches. Instrument manufacturers, consumable suppliers, and software developers are increasingly forming ecosystem agreements to bundle solutions that reduce integration friction for customers. Contract research organizations and clinical laboratories are expanding service portfolios to include long read offerings, which accelerates time to market for downstream applications and reduces the barrier for institutions that lack in-house capabilities.
Finally, adoption patterns have shifted toward application-driven deployment. Rather than blanket replacement of short read workflows, long read technologies are being applied where they deliver unique value: resolving structural variation, phasing haplotypes across complex loci, characterizing repeat expansions, and detecting epigenetic marks in native DNA. This pragmatic approach encourages hybrid workflows in which long reads complement short reads to deliver higher-confidence results while managing cost and throughput. Taken together, these shifts are altering investment priorities, operational models, and competitive dynamics across the long read ecosystem.
How 2025 tariff adjustments have driven supplier diversification, regional manufacturing shifts, and operational risk mitigation across long read sequencing supply chains
Trade policy and tariff adjustments have become material considerations for global supply chains that support long read sequencing, and the United States tariffs introduced in 2025 have a cumulative impact that extends beyond headline duty rates. These measures have accelerated vendor reassessments of sourcing strategies and prompted a reconfiguration of procurement timelines for instruments, reagents, and spare parts. Organizations with geographically concentrated supply chains have felt the operational implications most acutely, observing delays in delivery windows and a need to increase inventory buffers to insulate critical research and diagnostic activities from upstream tariff-induced variability.Because long read platforms rely on specialized components and reagents often manufactured across multiple jurisdictions, increased import costs have affected the landed price of both capital equipment and consumables. In response, several vendors have begun to adapt their commercial terms, offering more flexible service agreements, localized warranty support, and options for on-site reagent stocking to mitigate the financial uncertainty faced by customers. These vendor responses aim to preserve adoption momentum by reducing the immediate budgetary impact on end users while maintaining predictable supply relationships.
On a strategic level, the tariffs have intensified conversations about regionalization and supplier diversification. Research institutions and corporate R&D functions are evaluating alternative sourcing from markets that face fewer tariff frictions, and some manufacturers are accelerating localized production and assembly to maintain competitive pricing in affected regions. This reorientation carries longer-term implications for where future manufacturing capacity will be located and how quickly newer instrument generations can be ramped into global distribution.
Regulatory and procurement stakeholders must also grapple with the downstream effects of tariff-driven supply changes. When consumable substitution or instrument downtime becomes a possibility, validation plans and quality management systems require updated risk assessments and contingency protocols. For clinical laboratories operating under tight accreditation frameworks, the ability to demonstrate equivalence or to document controlled transitions between suppliers is essential. Therefore, the cumulative impact of tariffs in 2025 is not only financial but operational and regulatory, necessitating coordinated responses across procurement, technical, and compliance teams.
In-depth segmentation analysis explaining how products, technologies, applications, and end users converge to drive differentiated long read sequencing adoption and value
Segmentation analysis reveals where technical capabilities intersect with end-user needs and where investment is concentrated across the product, technology, application, and end user dimensions. From the product and service perspective, the ecosystem spans consumables, instruments, and software and services. Consumables extend into flow cells, kits, and reagents, which are foundational to run consistency and sample throughput. Instruments encompass sequencing systems and their accessories, and their design choices influence factors such as input fragment length, native DNA handling, and modularity for upgrades. Software and services include bioinformatics services, data analysis software, and maintenance services; together these offerings determine the velocity at which raw signal is converted into validated, actionable results for research and clinical decision-making.Across technology types, there are distinct performance and workflow considerations among nanopore sequencing, single-molecule real time sequencing, and synthetic long read sequencing. Nanopore sequencing is often chosen for its real-time data streaming and portability, enabling rapid sequencing in decentralized settings. Single-molecule real time approaches provide advantages in consensus accuracy and kinetic signal interpretation, which is particularly valuable for methylation and other epigenetic analyses. Synthetic long read strategies deliver long-range information by reconstructing long haplotypes from shorter reads, offering a bridge between throughput economics and structural resolution. Each technology demands specific investment in library preparation and informatics, and the selection typically reflects the balance between required read length, accuracy profile, and sample throughput.
Application segmentation highlights where long read capacities are translating into distinct value propositions. In agricultural genomics, resolving complex plant and animal genomes supports breeding programs and trait discovery. Cancer genomics benefits from the ability to detect structural variants and complex rearrangements that are sometimes missed by short read assays. Clinical research leverages long reads to elucidate disease mechanisms, develop novel biomarkers, and refine diagnostic assays. Microbial genomics relies on long reads to resolve plasmids, mobile elements, and full-length ribosomal operons necessary for robust pathogen characterization and antimicrobial resistance surveillance. Each application imposes unique demands on sample handling, depth of coverage, and validation rigor.
End users shape commercial dynamics through their operational priorities and regulatory constraints. Academic institutions often prioritize methodological flexibility and exploratory research, which favors platforms that are adaptable and cost-effective for low-to-moderate throughput studies. Clinical diagnostic laboratories require validated, reproducible workflows and robust vendor support to meet accreditation standards. Contract research organizations expand access for clients by offering scalable long read services and specialized expertise. Government and regulatory bodies drive standards and large-scale surveillance programs, emphasizing data quality and interoperability. Pharmaceutical and biotechnology companies apply long read sequencing across target discovery, biomarker validation, and cell line characterization, often integrating these data into broader translational pipelines. The alignment between vendor portfolios and these end user needs determines procurement patterns, partnership models, and investment in auxiliary services such as bioinformatics and maintenance.
Regional dynamics and commercialization nuances across the Americas, Europe Middle East & Africa, and Asia-Pacific that determine adoption speed and partnership strategies
Regional dynamics significantly influence technology adoption pathways, supply chain configurations, and partnership models across the long read sequencing ecosystem. In the Americas, strong networks of research universities, biotechnology firms, and clinical laboratories create a fertile environment for rapid technology evaluation and early adoption. The region’s robust venture and commercial infrastructure supports a vibrant vendor landscape and specialized service providers, which in turn accelerates the deployment of new instrument generations and application-specific workflows. Additionally, integrated domestic supply channels and a high concentration of contract research organizations help mitigate some import-related frictions by offering local reagent preparation and sequencing services.Europe, the Middle East and Africa present a mosaic of adoption patterns driven by regulatory frameworks, public health initiatives, and national investment in life sciences infrastructure. In several European markets, coordinated government programs and large-scale sequencing initiatives have supported the adoption of long read technologies for public health and clinical research. However, heterogeneous procurement rules and varying levels of local manufacturing capacity introduce complexity for vendors and end users seeking pan-regional consistency. In the Middle East and Africa, demand is often concentrated in centers of excellence and national reference laboratories, where long read sequencing is applied to infectious disease surveillance and genomic medicine projects that require robust, portable, and reproducible solutions.
Asia-Pacific combines substantial market heterogeneity with pockets of rapid uptake driven by significant public and private investment in genomics. Several countries in the region emphasize domestic biotech capacity building and have incentivized local production and research collaborations. This has led to heightened interest in technologies that offer scalability and cost-efficiency, as well as strong demand for integrated service offerings that simplify workflow adoption. Cross-border collaborations within the region and with partners in the Americas and EMEA further influence procurement patterns and the speed at which new methods are validated and commercialized.
Taken together, regional insights underscore the importance of tailored commercial strategies, region-specific validation packages, and flexible logistics solutions. Vendors that can align pricing models, support capabilities, and regulatory documentation with regional expectations will find it easier to reduce adoption friction and establish durable customer relationships across these diverse geographies.
Competitive and collaborative dynamics among instrument manufacturers, consumable suppliers, software vendors, and service providers that define commercial differentiation
A constellation of companies and specialized providers populate the long read sequencing ecosystem, each contributing through differentiated offerings in instruments, consumables, and computational services. Established instrument manufacturers continue to iterate on hardware platforms to improve throughput, accuracy, and ease of use, while a growing set of consumable suppliers focus on reagent stability, kit simplicity, and supply chain resilience. The interplay between instrument ecosystems and third-party consumable providers has become a competitive axis, as end users weigh the benefits of vertically integrated solutions against the flexibility of interchangeable consumables.In parallel, a new generation of software vendors and service organizations has emerged to address the bioinformatics and operational challenges inherent to long read data. These firms offer end-to-end pipelines, cloud-native analysis, and specialized interpretation modules for structural variant calling, epigenetic profiling, or microbial assembly. Service providers specializing in maintenance, warranty support, and on-site troubleshooting have likewise expanded their portfolios, recognizing that uptime and predictable performance are critical for clinical and high-throughput research operations.
Strategic partnerships and channel expansions are prevalent, with several firms entering collaboration agreements to deliver bundled solutions. Such alliances often reduce integration complexity for adopters and can accelerate time to validated data. Additionally, some organizations are investing in localized manufacturing or distribution agreements to address regional tariff and logistics uncertainties, thereby ensuring continuity of supply for critical reagents and spare parts.
For stakeholders evaluating potential partners, key considerations include the compatibility of instrument and consumable ecosystems, the maturity and regulatory readiness of software and analysis pipelines, the strength of technical support networks in target regions, and the flexibility of commercial terms to accommodate evolving workflow needs. Vendors that can demonstrate clear end-to-end support, robust validation documentation, and proactive supply chain strategies are more likely to secure enduring relationships with research, clinical, and commercial customers.
Actionable strategies for executives to align procurement, build genomic data capabilities, and fortify supply chains to accelerate sustainable long read sequencing integration
Industry leaders seeking to harness long read sequencing for strategic advantage should prioritize three interrelated courses of action: align procurement with downstream validation needs, invest in integrated bioinformatics and workforce capability, and build supply chain resilience through diversification and regional partnerships. First, procurement decisions should be made in the context of end-use validation pathways. Clinical and diagnostic entities must ensure that instrument choices and consumable dependencies are compatible with their accreditation timelines and analytical validation protocols. Research organizations should consider platform modularity to accommodate emergent application demands without disrupting established workflows.Second, leaders must invest in bioinformatics infrastructure and talent. The value of long read datasets depends on robust, reproducible analysis pipelines and the ability to interpret complex signals. Organizations should balance the use of commercial analysis suites with tailored in-house development to retain control over critical intellectual property and to ensure responsiveness to novel research questions. Upskilling laboratory and computational staff in long fragment handling, signal-level analysis, and variant interpretation reduces reliance on external partners and shortens time to insight.
Third, supply chain strategies must be rethought to manage tariff exposure, component scarcity, and logistical uncertainty. Diversifying suppliers, negotiating localized stocking arrangements, and pursuing regional distribution partnerships can materially reduce operational risk. Leaders should also formalize contingency plans that document validated alternatives for consumables and service pathways to maintain continuity in clinical and high-throughput research settings.
Finally, fostering cross-functional governance that includes R&D, procurement, regulatory affairs, and IT ensures that technology adoption proceeds with a comprehensive understanding of risk, cost, and performance. Such governance facilitates rapid decision-making when assay parameters or supplier conditions change, thereby preserving research timelines and clinical service levels while enabling strategic investment in long read capabilities.
Transparent description of the mixed-method research approach combining expert interviews, technical literature review, and cross-validation to ensure actionable and evidence-based insights
The research underpinning this analysis combined primary engagement with domain experts and secondary synthesis of peer-reviewed literature, technical white papers, regulatory documentation, and public statements from instrument and reagent providers. Primary inputs included structured interviews with laboratory directors, bioinformatics leads, procurement officers, and service providers operating across research, clinical, and industrial settings. These interviews explored operational priorities, validation challenges, procurement behaviors, and supplier performance considerations, providing qualitative context to observed technological and commercial shifts.Secondary research involved critical appraisal of technical publications that report on read accuracy, structural variant detection, and epigenetic measurement using long read platforms, as well as analysis of regulatory guidance documents and clinical accreditation standards relevant to genomic diagnostics. Particular attention was paid to reproducibility, validation practices, and workflow standardization to ensure that operational recommendations are grounded in documented best practices.
Analytical methods integrated cross-sectional synthesis of interview insights with thematic content analysis of technical sources to identify recurring drivers, pain points, and enablers across product, technology, application, and end user dimensions. Regional dynamics and tariff impacts were assessed through a combination of stakeholder testimony and review of public policy changes, logistics reports, and vendor responses. Where vendor statements or manufacturer documentation addressed supply chain remedies or localized production, these were corroborated with third-party logistics insights to evaluate practical feasibility.
Throughout the research process, methodological rigor was maintained by triangulating claims across multiple independent sources, documenting assumptions underlying operational assessments, and flagging areas where evidence remains emergent and requires continued monitoring. This approach ensures that conclusions reflect both current realities and near-term trajectories relevant to decision-makers in long read sequencing.
A concise synthesis of how technological strengths, operational challenges, and strategic planning converge to guide successful long read sequencing adoption
Long read sequencing is at an inflection point where technological improvements, ecosystem maturation, and shifting commercial imperatives are converging to broaden practical applications. The technology’s unique capacity to resolve structural complexity and epigenetic signals positions it as a critical complement to existing genomic toolsets, especially in applications that demand contiguous context and phase information. At the same time, operational challenges including consumable reliability, bioinformatics maturity, and supply chain exposure require deliberate strategies to realize the full scientific and clinical value.Decision-makers should therefore view long read adoption as a phased journey that combines targeted application deployment, investment in analytical capability, and proactive supplier management. By prioritizing validation pathways that align with organizational goals and by cultivating partnerships that reduce integration friction, institutions can extract disproportionate value from long read data while managing cost and operational risk. Continued monitoring of policy changes, vendor roadmaps, and peer-reviewed performance studies will be essential to adapt strategies as technologies and market dynamics evolve.
In summary, the opportunities afforded by long read sequencing are substantial, but they are best captured through a deliberate, interdisciplinary approach that balances technological ambition with pragmatic attention to validation, data interpretation, and supply resilience.
Additional Product Information:
- Purchase of this report includes 1 year online access with quarterly updates.
- This report can be updated on request. Please contact our Customer Experience team using the Ask a Question widget on our website.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
16. China Long Read Sequencing Market
Companies Mentioned
The key companies profiled in this Long Read Sequencing market report include:- Arima Genomics, Inc.
- BGI Genomics Co., Ltd.
- Dovetail Genomics, LLC
- Element Biosciences, Inc.
- F. Hoffmann-La Roche Ltd.
- Genapsys, Inc.
- Hitachi High-Technologies Corporation
- Illumina, Inc.
- Nabsys, Inc.
- Nuclera Nucleics Ltd.
- Oxford Nanopore Technologies Limited
- Pacific Biosciences of California, Inc.
- Phase Genomics, Inc.
- QIAGEN N.V.
- Quantapore, Inc.
- Takara Bio Inc.
- Thermo Fisher Scientific Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 189 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
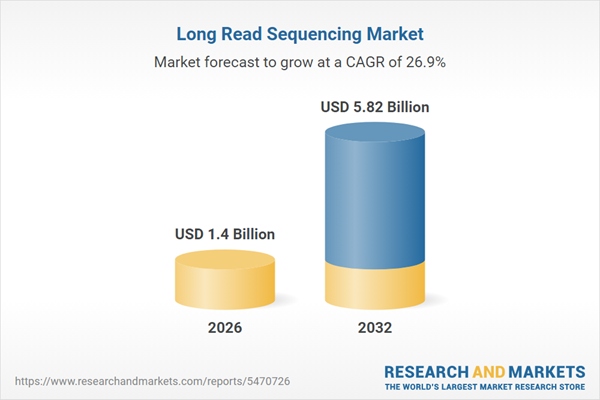
| Estimated Market Value ( USD | $ 1.4 Billion |
| Forecasted Market Value ( USD | $ 5.82 Billion |
| Compound Annual Growth Rate | 26.9% |
| Regions Covered | Global |
| No. of Companies Mentioned | 18 |