Global Medical Device Outsourcing Market - Key Trends & Drivers Summarized
What Does Medical Device Outsourcing Entail, and Why Is It Becoming a Necessity?
Medical device outsourcing refers to the practice of hiring third-party companies to perform functions ranging from product design and manufacturing to testing, validation, and regulatory consultancy services for medical device companies. This trend has grown as companies seek to reduce costs and accelerate time to market amidst increasing regulatory complexities. Outsourcing partners not only provide specialized expertise in various facets of medical device production but also offer economies of scale, which can be particularly advantageous for smaller manufacturers or startups lacking the infrastructure or capital to perform these tasks in-house. Furthermore, these third-party firms stay abreast of regulatory changes and standards across different markets, ensuring that medical devices comply with local and international laws, thus safeguarding manufacturers against potential legal issues.How Has Outsourcing Transformed the Medical Device Industry?
In recent years, how has the adoption of outsourcing reshaped the landscape of the medical device industry? As medical technology evolves, the demand for more sophisticated, cost-effective, and efficiently manufactured products escalates. Outsourcing addresses these needs by enabling device companies to leverage external engineering and manufacturing capabilities, thereby focusing more on their core competencies such as R&D and market expansion strategies. Moreover, outsourcing firms often bring innovative manufacturing techniques and materials to the table, which helps device companies stay at the forefront of technological advancements without bearing the full cost of new equipment or specialist training for their staff.Who Are the Key Players in Medical Device Outsourcing, and What Services Do They Offer?
Who dominates the medical device outsourcing market, and what range of services do these entities typically provide? The market is populated by a mix of full-service providers who offer an end-to-end solution, from initial concept development through to final product delivery, and niche firms that specialize in specific segments such as regulatory compliance, clinical trials management, or particular stages of manufacturing. These providers cater to a diverse array of needs within the medical device sector, including but not limited to cardiovascular devices, orthopedic devices, and diagnostic imaging. Their services help medical device companies not only minimize operational costs but also improve product quality and safety, enhancing overall market competitiveness.What Are the Key Growth Drivers in the Medical Device Outsourcing Market?
The growth in the medical device outsourcing market is driven by several factors. The primary catalyst is the increasing complexity of medical devices combined with stringent regulatory standards that necessitate specialized knowledge in both development and compliance procedures. As medical devices incorporate more advanced technologies such as robotics, AI, and connected IoT features, the expertise required to develop and maintain these sophisticated systems often surpasses the capabilities of in-house teams, particularly at smaller firms. Additionally, the rising prevalence of chronic diseases globally fuels the demand for medical devices, further compelling companies to accelerate development and commercialization to capitalize on market opportunities. The economic advantage of outsourcing also remains a significant driver, as it allows companies to avoid the high costs associated with expanding internal manufacturing capabilities and maintaining compliance with evolving regulations. The global expansion of healthcare markets, especially in emerging economies with increasing healthcare expenditures, offers additional growth opportunities for outsourcing firms, enabling them to establish a presence and offer localized services that cater to regional regulatory and market demands.Report Scope
The report analyzes the Medical Device Outsourcing market, presented in terms of market value (USD). The analysis covers the key segments and geographic regions outlined below.- Segments: Service (Contract Manufacturing, Quality Assurance, Product Testing & Sterilization Services, Product Design & Development Services, Regulatory Affairs Services, Other Services); Application (Cardiovascular Devices, IVD Devices, Diagnostic Imaging Devices, Orthopedic Devices, Ophthalmic Devices, Drug Delivery Devices, Other Applications).
- Geographic Regions/Countries: World; USA; Canada; Japan; China; Europe; France; Germany; Italy; UK; Spain; Russia; Rest of Europe; Asia-Pacific; Australia; India; South Korea; Rest of Asia-Pacific; Latin America; Argentina; Brazil; Mexico; Rest of Latin America; Middle East; Iran; Israel; Saudi Arabia; UAE; Rest of Middle East; Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Cardiovascular Devices Application segment, which is expected to reach US$104.2 Billion by 2030 with a CAGR of 9.8%. The IVD Devices Application segment is also set to grow at 10.8% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $55.5 Billion in 2024, and China, forecasted to grow at an impressive 10.3% CAGR to reach $24 Billion by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Medical Device Outsourcing Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Medical Device Outsourcing Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Medical Device Outsourcing Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as B. Braun Melsungen AG, Cardinal Health, Inc., HCL Technologies Ltd., Charles River Laboratories International, Inc., Heraeus Holding GmbH and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 74 companies featured in this Medical Device Outsourcing market report include:
- B. Braun Melsungen AG
- Cardinal Health, Inc.
- HCL Technologies Ltd.
- Charles River Laboratories International, Inc.
- Heraeus Holding GmbH
- Eurofins Scientific SE
- Benchmark Electronics, Inc.
- Celestica, Inc.
- Clinipace Inc.
- Conductive Technologies, Inc.
- Dravon Medical, Inc.
- Cadence, Inc.
- Freyr, Inc.
- Global Life Sciences Solutions USA LLC (Cytiva)
- ICON plc
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- B. Braun Melsungen AG
- Cardinal Health, Inc.
- HCL Technologies Ltd.
- Charles River Laboratories International, Inc.
- Heraeus Holding GmbH
- Eurofins Scientific SE
- Benchmark Electronics, Inc.
- Celestica, Inc.
- Clinipace Inc.
- Conductive Technologies, Inc.
- Dravon Medical, Inc.
- Cadence, Inc.
- Freyr, Inc.
- Global Life Sciences Solutions USA LLC (Cytiva)
- ICON plc
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 366 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
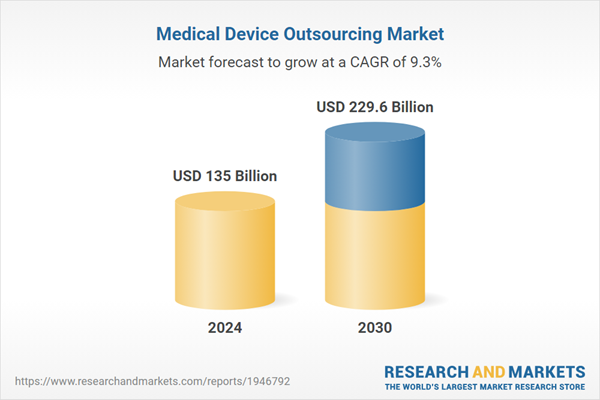
| Estimated Market Value ( USD | $ 135 Billion |
| Forecasted Market Value ( USD | $ 229.6 Billion |
| Compound Annual Growth Rate | 9.3% |
| Regions Covered | Global |