Speak directly to the analyst to clarify any post sales queries you may have.
10% Free customizationThis report comes with 10% free customization, enabling you to add data that meets your specific business needs.
Key Market Drivers
Rising Demand for Cost-Effective Solutions
The rising need for cost-effective production strategies is a primary force fueling the global medical device outsourcing market. As healthcare expenditures escalate worldwide, manufacturers are under pressure to maintain high product quality while cutting operational costs. Outsourcing offers a viable solution, allowing OEMs to delegate non-core tasks such as product design, manufacturing, testing, and packaging to third-party experts, often in cost-effective regions. This shift reduces capital investment in facilities, labor, and technology, enabling companies to focus on R&D and innovation. In addition to reducing costs, outsourcing accelerates product development cycles and time-to-market, especially important in highly competitive markets. Furthermore, in cost-sensitive emerging economies, outsourcing helps deliver affordable medical devices without compromising on safety or standards, thereby increasing market accessibility and driving strategic collaborations across the industry.Key Market Challenges
Intellectual Property (IP) Risks
Intellectual property protection remains a critical challenge in the global medical device outsourcing landscape. As OEMs outsource core functions, they often share proprietary technologies and designs with external partners, raising concerns over IP theft and unauthorized replication. This is particularly concerning in regions with limited regulatory enforcement or weaker IP laws.Infringements can result in financial losses, reputational harm, and reduced market competitiveness. Furthermore, disagreements over ownership rights and licensing can delay product development and lead to legal disputes. To mitigate these risks, companies must adopt stringent contractual frameworks, enforce non-disclosure agreements, and carefully vet outsourcing partners for compliance with international IP standards. Vigilance in IP management is vital for sustaining innovation and securing competitive advantage in the outsourcing ecosystem.
Key Market Trends
Expansion of Analytical Testing Services
The growing complexity of medical devices has increased the demand for advanced analytical testing services, a key trend shaping the global medical device outsourcing market. Regulatory authorities like the FDA are intensifying scrutiny, especially for emerging technologies such as AI-enabled devices. In 2023 alone, the FDA approved 221 AI-powered devices, up from just six in 2015. This surge requires rigorous testing to ensure safety and effectiveness, propelling the need for outsourced analytical expertise.Additionally, adherence to global standards such as ISO 13485 - which had over 20,000 valid certifications globally by the end of 2023 - further underscores the critical role of robust quality management systems. Outsourcing analytical testing allows manufacturers to access specialized labs, reduce compliance risks, and maintain consistent quality throughout the product lifecycle. This trend is expected to gain momentum as medical devices become increasingly sophisticated and regulated.
Key Market Players
- Laboratory Corporation of America Holdings
- Pace Analytical Services, Inc.
- Intertek Group plc
- Charles River Laboratories
- Medical Device Testing Services
- PAREXEL International Corporation
- Accell Clinical Research, LLC
- Criterium, Inc.
- ICON plc.
- IQVIA Inc.
- Integer Holdings Corporation
Report Scope:
In this report, the Global Medical Device Outsourcing Market has been segmented into the following categories, in addition to the industry trends which have also been detailed below.Medical Device Outsourcing Market, By Service:
- Quality Assurance
- Regulatory Affairs Services
- Product Design and Development Services
- Product Testing & Sterilization Services
- Product Implementation Services
- Product Upgrade Services
- Product Maintenance Services
- Contract Manufacturing
Medical Device Outsourcing Market, By Application:
- Cardiology
- Diagnostic Imaging
- Orthopedic
- IVD
- Ophthalmic
- General and Plastic Surgery
- Drug Delivery
- Dental
- Endoscopy
- Diabetes Care
- Others
Medical Device Outsourcing Market, By Class:
- Class I
- Class II
- Class III
Medical Device Outsourcing Market, By Region:
- North America
- United States
- Canada
- Mexico
- Europe
- France
- United Kingdom
- Italy
- Germany
- Spain
- Asia-Pacific
- China
- India
- Japan
- Australia
- South Korea
- South America
- Brazil
- Argentina
- Colombia
- Middle East & Africa
- South Africa
- Saudi Arabia
- UAE
Competitive Landscape
Company Profiles: Detailed analysis of the major companies present in the Global Medical Device Outsourcing Market.Available Customizations:
With the given market data, the publisher offers customizations according to a company's specific needs. The following customization options are available for the report.Company Information
- Detailed analysis and profiling of additional market players (up to five).
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
- Laboratory Corporation of America Holdings
- Pace Analytical Services, Inc.
- Intertek Group plc
- Charles River Laboratories
- Medical Device Testing Services
- PAREXEL International Corporation
- Accell Clinical Research, LLC
- Criterium, Inc.
- ICON plc.
- IQVIA Inc.
- Integer Holdings Corporation
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 184 |
| Published | April 2025 |
| Forecast Period | 2024 - 2030 |
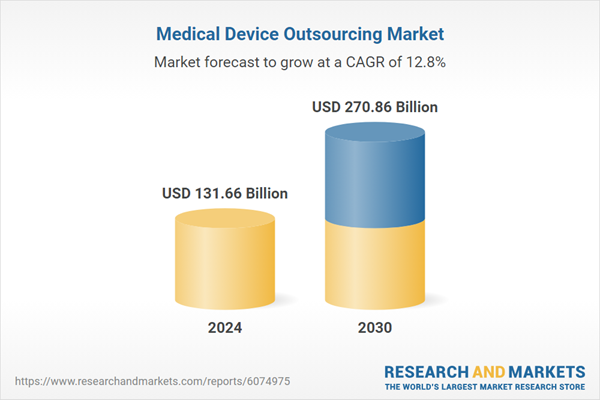
| Estimated Market Value ( USD | $ 131.66 Billion |
| Forecasted Market Value ( USD | $ 270.86 Billion |
| Compound Annual Growth Rate | 12.7% |
| Regions Covered | Global |
| No. of Companies Mentioned | 11 |