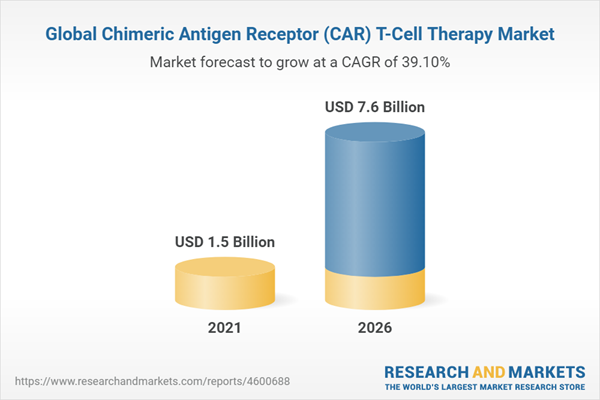
The global market for chimeric antigen receptor (CAR) T-cell therapy is estimated to grow from $1.5 billion in 2021 to reach $7.6 billion by 2026, at a compound annual growth rate (CAGR) of 39.1% during 2021-2026.
The North American market for CAR T-cell therapy is estimated to grow from $751.5 million in 2021 to reach $3.5 billion by 2026, at a CAGR of 35.8% during 2021-2026.
The Asia-Pacific market for CAR T-cell therapy is estimated to grow from $228.9 million in 2021 to reach $2.1 billion by 2026, at a CAGR of 56.0% during 2021-2026.
Report Scope:
The key objectives of this study are to -
- Review the historical development of CAR T-cell technology.
- Discuss the principles of chimeric antigen receptor design.
- Understand the mechanisms of action associated with CAR T-cell immunotherapy.
- Highlight the ongoing clinical and non-clinical advancements in the field of CAR T-cell therapy.
- Access the side effects, disadvantages and limitations of existing CAR T-cell technologies.
- Examine the currently marketed drugs, including development activities and details of patent expirations.
- Determine the production methods for CAR T-cells.
- Review the range of joint ventures, collaborations, license and research agreements currently focused on CAR T-cell technology.
- Review of the regulatory requirements.
- Review the global CAR T-cell therapy market dynamics.
- Survey the key players involved in the development of therapeutics for CAR T-cell immunotherapy and provide company profiles.
By purchasing this study, the reader will gain -
- An improved understanding of the current state and future of this exciting, new and innovative technology.
- The latest information on the leading companies engaged in developing this technology, clinical trials being conducted, a review of the status of their intellectual property, product pipelines and insight into their proprietary technologies.
- The role and influence of individual countries relating to the development of CAR T-cell therapy and the number of CAR T-cell trials in the U.S. versus China and other countries.
- Knowledge of the market potential for the CAR T-cell therapy market and anticipated development of the market.
The analysis includes the use of charts and graphs measuring product growth and trends within the marketplace. In addition, an analysis of the incidence and mortality associated with cancers and the target market helps provide the reader with a deeper understanding of the possibilities for future treatment and avenues for possible R&D budgets. Company-specific information, including sales figures, product pipeline status and R&D trends, is provided throughout the report.
The Report Includes:
- 37 data tables and 55 additional tables
- An updated review of the global market for chimeric antigen receptor (CAR) T-cell therapy with emphasis on the current research and development status
- Analyses of the global market trends, with historic data from 2018-2020, estimates for 2021 and 2022, and projections of compound annual growth rates (CAGRs) through 2026
- Evaluation and forecast the overall CAR T-Cell therapy market size in dollar value terms, and corresponding market share analysis by product, application, technology and region
- Highlights of the market potential for the CAR T-cell therapy market, opportunities and trends estimating current and future demand, and impact of COVID-19 on the progress of this market
- Assessment of currently marketed drugs, including development activities, R&D activities and anticipated developments, along with a look into the patent expirations within the industry
- Latest information on the major stakeholders of the global CAR T-Cell therapy market, along with a review of their intellectual property status, product innovations, technological advancements, and research collaborations and business consolidations
- Insight into the current competitive environment, recent mergers and acquisitions, license agreements, and company revenue share analysis of the key players involved in the development of therapeutics for CAR T-cell therapy
- Descriptive company profiles of the leading industry players, including AstraZeneca, Bristol Myers Squibb, Gilead Sciences, Novartis AG, F. Hoffmann-La Roche and Takeda Pharmaceutical Co., Ltd.
Table of Contents
Chapter 1 Introduction
- Scope of This Report
- What's New in this Update?
- Methodology and Information Sources
- Primary Data and Information Gathering
- Secondary Data and Information Gathering
- Market Share Analysis and Market Forecast
- Analyst's Credentials
- Custom Research
- Related Reports
Chapter 2 Summary and Highlights
- CAR T-Cell Design and Evolution
- FDA Product Approvals
- Market Analysis
- Clinical Applications of CAR T-Cell Therapy
- CARs for Tumors of the Hematopoietic and Lymphoid Tissues
- CAR T-Cell Therapy for Solid Tumors
- Barriers to Research and Product Development
- Clinical Trials
Chapter 3 Introduction to Chimeric Antigen Receptor (CAR) T-Cell Therapy
- Introduction
- A Brief History of CAR T-Cell Therapy Development
- Antigens
- Exogenous Antigens
- Endogenous Antigens
- Autoantigens
- Tumor Antigens
- Cluster of Differentiation
- Lymphocytes
- B Cells
- T Cells
- Adoptive Cell Transfer (ACT) Technologies
- Tumor-Infiltrating Lymphocytes (TILs)
- T-Cell Receptors (TCRs)
- Chimeric Antigen Receptors (CARs)
Chapter 4 Impact of COVID-19 on the Market
- Overview
- Outlook
Chapter 5 Market Dynamics
- Market Dynamics
- Selected Drivers of the Global CAR T-Cell Market
- Trends
- Selected Restraints of the Global CAR T-Cell Market
Chapter 6 Current Production Methods, Latest Technological Advances and Future Direction
- Production of CAR T Cells
- Stage 1: Leukapheresis and T-Cell Isolation
- Stage 2: T-Cell Activation, Transduction and Modification of CAR T Cells
- Stage 3: Expanding Modified CAR T Cells
- Overcoming CAR T-Cell Manufacturing Challenges
- Latest Advances in Production Processes
- Closed, Automated Production Systems
- End-to-End Production Systems and Solutions
- Key Technologies Used in the Manufacturing Stage
Chapter 7 Clinical Trials on CAR T Cells and Related Technologies
- Introduction
- Clinical Trials Being Conducted Globally
- Distribution of CAR T-Cell Trials in the U.S.
- Distribution of CAR T-Cell Clinical Trials in China
Chapter 8 Cancer Demographics: A Global Summary
- Cancer: The Disease
- Epidemiology by Cancer Type/Site
- Global Cancer Incidence
- Global Cancer Prevalence, 2020
- Global Cancer Mortality Overview
- Cancer Epidemiology by Region
- Europe
- North America
- Asia-Pacific
- Rest of the World
- Global Cancer Burden and Worldwide Cancer Risk Factors
- Tobacco Use and Statistics
- Alcohol Abuse and Cancer Statistics
- Obesity and Cancer Statistics
- Infectious Diseases and Cancer
- Inherited Genes: Diseases and Cancer
- Sun, Ultraviolet Radiation and Cancer
- Hormones and Cancer
Chapter 9 Global CAR T-Cell Market Analysis
- Introduction
- Global CAR T-Cell Market
- Yescarta
- Tecartus
- Kymriah
- Breyanzi
- Abecma
Chapter 10 Regional Analysis
- North America
- Europe
- Asia-Pacific
- Rest of the World
Chapter 11 Regulatory and Legislative Requirements
- North America
- United States
- Canada
- Europe
- Mutual Recognition Procedure (MRP)
- Decentralized Procedure (DCP)
- Centralized Procedure (CP)
- Asia-Pacific
- Japan
- China
- India
- South Korea
- Central and South America
- Brazil
- Argentina
- Mexico
- Middle East and Africa
- South Africa
- Saudi Arabia
- Published Guidelines on Production and Testing of Cell-Based Therapies
Chapter 12 Patent Review/New Developments
- Introduction
- Complexities of the Patents and Patent Applications for Biotechnology and Pharmaceutical Companies
- Anticipated Increase in Patent Litigation on Customized Patient Treatments
- Company Specific Intellectual Property and Patent Information
- Amgen
- Avacta Life Sciences Ltd.
- Bellicum Pharmaceuticals Inc.
- Bluebird Bio
- Celgene Corp.
- Cellectis
- Celyad SA
- Editas Medicine Inc.
- Eureka Therapeutics Inc.
- Gilead Sciences Inc.
- iCell Gene Therapeutics
- Juno Therapeutics Inc. (A Celgene Co.)
- Mustang Bio Inc.
- Noile-Immune Biotech
- Novartis AG
Chapter 13 Global Competitive Market Landscape
- Global CAR T-Cell Market Share Analysis
- Market Share Analysis
- Product Launches
- Strategic Initiatives
- Mergers and Acquisitions
Chapter 14 Company Profiles
- Abbvie Inc.
- Amgen
- AstraZeneca
- Avacta Life Sciences Ltd.
- Bellicum Pharmaceuticals
- Bluebird Bio
- Bristol Myers Squibb
- Cellectis
- Celyad SA
- Editas Medicine Inc.
- Eureka Therapeutics Inc.
- Formula Pharmaceuticals Inc.
- Gilead Sciences
- F. Hoffman-La Roche AG
- Icell Gene Therapeutics
- Mustang Bio Inc.
- Noile-Immune Biotech
- Novartis AG
- Protheragen Inc.
- Puretech Health
- Servier Laboratories
- Takeda Pharmaceuticals
- Transgene SA
Chapter 15 Appendix A
- Company Contact Details
Chapter 16 Appendix B
- Government Regulatory Agencies and Professional Organizations
Chapter 17 Appendix C
- Commonly Used Acronyms Associated with CAR T-Cell Therapy
Chapter 18 Appendix D
- Recent Patent Applications Filed Related to CAR T-Cell Therapy
Chapter 19 Appendix E
- CAR T-Cell and CD19 Clinical Trials in China
List of Tables
Summary Table A: Global CAR T-Cell Market, by Region, Through 2026
Summary Table B: Global CAR T-Cell Market, by Product, Through 2026
Table 1: Tumor-Associated Antigens of CAR T-Cell Targets
Table 2: List of Relevant Human Clusters of Differentiation
Table 3: Classification of Natural Killer T Cells
Table 4: Summary Classification of Tumors of the Hematopoietic and Lymphoid Tissues
Table 5: Tumor-Associated Antigens of CAR T-Cell Target
Table 6: Reported COVID-19 Confirmed Cases and Deaths, by Country, October 22, 2021
Table 7: Supplementary Components in CAR T-Cell Manufacture
Table 8: Technologies Used in the Selection, Isolation and Enrichment of Cells During the Manufacture of CAR T Cells
Table 9: Technologies Used in the Activation and Simulation of Cells During the Manufacture of CAR T Cells
Table 10: Technologies Used in the Gene Transfer and Delivery of Cells During the Manufacture of CAR T Cells
Table 11: Gene Transfer and Gene Delivery Technologies Developed by Poseida Therapeutics Inc.
Table 12: Technologies Used in the Expansion and Culture of Cells During the Manufacture of CAR T Cells
Table 13: Technologies Used in the Formulation of Cells During the Manufacture of CAR T Cells
Table 14: Technologies Used in the Cryopreservation of Cells During the Manufacture of CAR T Cells
Table 15: Technologies Used in the Thawing of Cells During the Manufacture of CAR T Cells
Table 16: Emerging CAR T-Cell Therapy Clinical Trials, by Company
Table 17: Global CAR T-Cell Clinical Trial Activity, by Study Location/Region, as of Oct. 2021
Table 18: Clinical Trials on CD19 Directed CAR T-Cells in the U.S.
Table 19: Examples of Clinical Trials on CD19-Directed CAR T Cells in China
Table 20: Examples of Clinical Trials on CAR T Cells Targeting Non-CD19 Antigens in China
Table 21: Examples of Clinical Trials on CAR T Cells for Solid Tumors in China
Table 22: Clinical Trials on CAR T Cells with Fourth-Generation CARs in China
Table 23: Global Cancer Incidence, by Cancer Type/Site, 2020
Table 24: Global Cancer Mortality, by Cancer Type/Site, 2020
Table 25: Estimated Number of New Cancer Cases in the U.S., by Cancer Type/Site and Sex, 2021
Table 26: Estimated Number of Cancer Deaths in the U.S., by Cancer Type/Site and Sex, 2021
Table 27: Global CAR T-Cell Market, by Product, 2026
Table 28: Global CAR T-Cell Market, by Region, Through 2026
Table 29: Global CAR T-Cell Market Share, by Region, 2021
Table 30: North American CAR T-Cell Market, Through 2026
Table 31: North American CAR T-Cell Market, by Product, Through 2026
Table 32: European CAR T-Cell Market, Through 2023
Table 33: European CAR T-Cell Market, by Product, Through 2026
Table 34: Asia-Pacific CAR T-Cell Market, Through 2023
Table 35: Asia-Pacific CAR T-Cell Market, by Product, Through 2026
Table 36: Rest of the World CAR T-Cell Market, Through 2020-2026
Table 37: Rest of the World CAR T-Cell Market, by Product, Through 2026
Table 38: Overview of Healthcare System in India
Table 39: Recent CAR T-Cell Therapy Patents and Patent Applications, 2020-2021
Table 40: Recent Bluebird Bio Patents and Patent Applications, 2010-Apr. 2021
Table 41: Recent Celgene Patents and Patent Applications, 2018-2021
Table 42: Recent Cellectis Patents and Patent Applications, 2018-2021
Table 43: Editas Medicine Patent Applications, 2010-2021
Table 44: Eureka Therapeutics Patent Applications, 2015-2021
Table 45: Kite Pharma’s (Gilead Sciences Subsidiary) Core Anticancer Technology: Yeda Research and Development Co., Ltd. Patents and Patent Applications, 1993-Apr. 2021
Table 46: iCell Gene Therapeutics Patent Applications
Table 47: Juno Therapeutics Inc. and Memorial Sloan Kettering Patents and Patent Applications
Table 48: Mustang Bio and City of Hope National Medical Center Patents and Patent Applications
Table 49: Noile-Immune Biotech Inc. Yamaguchi University Patent Applications
Table 50: Novartis AG and University of Pennsylvania Patents and Patent Applications, 2013-2021
Table 51: Global CAR T-Cell Market Share, by Company, 2021
Table 52: Global CAR T-Cell Therapy Market, by Company Revenue, 2018-2021
Table 53: New Product Launches in the CAR T-Cell Therapy Market
Table 54: New Strategic Initiatives in CAR T-Cell Therapy Market
Table 55: Mergers and Acquisitions in the CAR T-Cell Therapy Market, 2018-2021
Table 56: Regulatory Approvals in the CAR T-Cell Therapy Market, 2018-2021
Table 57: AbbVie Inc.: Marketed Products
Table 58: AbbVie Inc.: Net Revenue, 2017-2020
Table 59: AbbVie Inc.: Key Developments
Table 60: Amgen: Financials, 2014-2020
Table 61: Amgen: Financials, by Product, 2014-2020
Table 62: Avacta Group plc: Financials, 2015-2017
Table 63: Bellicum Pharmaceuticals Inc.: Financials, 2019 and 2020
Table 64: Bellicum Pharmaceuticals Inc.: Key Switch Technologies
Table 65: Bluebird Bio: Financials, 2018-2020
Table 66: Bluebird Bio: Key Developments
Table 67: Bristol-Myers Squibb: Net Revenue, 2017-2020
Table 68: Bristol-Myers Squibb: Key Cancer Biologics Product Portfolio
Table 69: Bristol-Myers Squibb: Key Developments
Table 70: Juno Therapeutics: Product Pipeline
Table 71: Editas Medicine Inc.: Financials, 2015-2020
Table 72: Editas Medicine Inc.: Key Developments
Table 73: Gilead Sciences: Financials, 2014-2020
Table 74: Gilead Sciences: Key CAR T-Cell Therapies
Table 75: Gilead Sciences: Individual Product Sales, 2018-2020
Table 76: Gilead Sciences: Product Pipeline
Table 77: F. Hoffman-La Roche: Financials, 2019 and 2020
Table 78: F. Hoffman-La Roche: Pharmaceutical Division Financials, 2019 and 2020
Table 79: F. Hoffman-La Roche: Total Revenue, by Geography, 2020
Table 80: iCell Gene Therapeutics: Product Development Pipeline
Table 81: Mustang Bio Inc.: Research and Development Pipeline
Table 82: Novartis AG: Revenues, by Segment, 2019 and 2020
Table 83: Protheragen: Enabling Technologies
Table 84: PureTech Health: Immune System Pipeline
Table 85: Takeda Pharmaceuticals: Financials, 2014-2016
Table 86: Company Contact Details
Table 87: Government Regulatory Agencies and Professional Organizations Associated with CAR T-Cell Therapy
Table 88: Commonly Used Acronyms Associated with CAR T-Cell Therapy
Table 89: Recent Patent Applications Filed Related to CAR T-Cell Therapy
Table 90: CAR T-Cell and CD19 Clinical Trials in China
List of Figures
Summary Figure A: Global CAR T-Cell Market Share, by Region, 2021
Summary Figure B: Global CAR T-Cell Market, by Product, 2018-2026
Summary Figure C: Global CAR T-Cell Clinical Trials, by Phase, as of October 2021
Figure 1: Historical Development of CAR T-Cell Therapy
Figure 2: Cytotoxic T-Cell Activation and Action
Figure 3: Overview of Signal Transduction Pathways Involved in Apoptosis
Figure 4: Model for Th Differentiation from Naïve CD4+ T Cells
Figure 5: Diagram of Regulatory T Cell, Effector T Cells and Dendritic Cells Showing Putative Mechanisms of Suppression by Regulatory T Cells
Figure 6: Interactions and Cross-Talk Between Different Subsets of NKT Cells and Other Immune Cells in TME
Figure 7: Schematic of Adoptive Cell Transfer Therapies
Figure 8: Structure and Function of the TCR
Figure 9: General Structure of a Chimeric Antigen Receptor
Figure 10: CAR T-Cell Design
Figure 11: CAR T-Cell Manufacture and Product Testing Process
Figure 12: Sleeping Beauty (SB) Transposon System Mechanism Schematic
Figure 13: PiggyBac (PB) Transposon System “Cut and Paste” Mechanism Schematic
Figure 14: CRISPR/Cas9 System Mechanism Schematic
Figure 15: Global CAR T-Cell Clinical Trial Activity, by Phase, as of Oct. 2021
Figure 16: Global CAR T-Cell Clinical Trial Activity, by Study Start Date and Phase, 2005-Oct. 2021
Figure 17: Distribution Map of CAR T-Cell Clinical Trial Activity Globally, by Study Location/Country, as of Oct. 2021
Figure 18: Distribution Map of CAR T-Cell Clinical Trial Activity in Europe, by Study Location/Country, as of Oct. 2021
Figure 19: Distribution Map of Phase I CAR T-Cell Clinical Trial Activity Globally, by Study Location/Country, as of Oct. 2021
Figure 20: Distribution Map of Phase II CAR T-Cell Clinical Trial Activity Globally, by Study Location/Country, as of Oct. 2021
Figure 21: Distribution Map of Phase III CAR T-Cell Clinical Trial Activity Globally, by Study Location/Country, as of Oct. 2021
Figure 22: Distribution Map of CAR T-Cell Clinical Trial Activity in the U.S., by Study Location, as of Oct. 2021
Figure 23: Distribution of CAR T-Cell Clinical Trials in China, South Korea and Taiwan, by Study Location/Region, as of Oct. 2021
Figure 24: CAR T-Cell Clinical Trials in China, by Study Start Date and Phase, 2006-Oct. 2021
Figure 25: Global Cancer Incidence, by Sex and Cancer Type/Site, 2020
Figure 26: Global Cancer Incidence, by Region, 2020
Figure 27: Global Cancer Incidence Trends, by Cancer Type/Site, 2020-2030
Figure 28: Global Cancer Prevalence by Cancer Type/Site and Sex, 2020
Figure 29: Global CAR T-Cell Market, by Product, 2018-2026
Figure 30: Global Market for Yescarta Cell Therapy, by Region, 2017-2021
Figure 31: Global Market Shares of Yescarta Cell Therapy, by Region, 2020
Figure 32: Global Market for Tecartus, by Region, 2020-2021
Figure 33: Global Market Shares of Tecartus, by Region, 2020
Figure 34: Global Market for Kymriah, by Region, 2017-2021
Figure 35: Global Market Shares of Kymriah, by Region, 2020
Figure 36: Global CAR T-Cell Market Share, by Region, 2021
Figure 37: Drug Discovery and Development Timeline
Figure 38: Mutual Recognition Procedure
Figure 39: Decentralized Procedure
Figure 40: Japanese Drug Funding/Reimbursement Approval Procedure
Figure 41: The Chinese Healthcare System
Figure 42: Argentina: Regulatory Drug Approval Procedure Standard Review 120-240 Business Days* for Approval**
Figure 43: Current Structure of the Health Care Sector in Saudi Arabia
Figure 44: Global CAR T-Cell Therapy Market Share, by Company, 2021
Figure 45: AbbVie Inc.: Revenue Share, by Country, 2020
Figure 46: Bellicum Pharmaceuticals Inc.: Product Pipeline
Figure 47: Bristol-Myers Squibb: Revenue Share, by Segment, 2020
Figure 48: Bristol-Myers Squibb: Revenue Share, by Region, 2020
Figure 49: Cellectis: Allogeneic CAR-T Pipeline
Figure 50: Eureka Therapeutics Inc.: Product Pipeline
Figure 51: Formula Pharmaceuticals Inc.: Product Pipeline
Figure 52: Kite Pharma Inc. (a Subsidiary of Gilead Sciences Inc.): CAR and TCR Pipeline
Figure 53: Noile-Immune Biotech: CAR Pipeline
Figure 54: Novartis AG: Sales Prediction for Kymriah
Figure 55: Protheragen: Product Pipeline
Companies Mentioned
- Abbvie Inc.
- Amgen
- AstraZeneca
- Avacta Life Sciences Ltd.
- Bellicum Pharmaceuticals
- Bluebird Bio
- Bristol Myers Squibb
- Cellectis
- Celyad SA
- Editas Medicine Inc.
- Eureka Therapeutics Inc.
- F. Hoffman-La Roche AG
- Formula Pharmaceuticals Inc.
- Gilead Sciences
- Icell Gene Therapeutics
- Mustang Bio Inc.
- Noile-Immune Biotech
- Novartis AG
- Protheragen Inc.
- Puretech Health
- Servier Laboratories
- Takeda Pharmaceuticals
- Transgene SA
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 353 |
| Published | December 2021 |
| Forecast Period | 2021 - 2026 |
| Estimated Market Value ( USD | $ 1.5 Billion |
| Forecasted Market Value ( USD | $ 7.6 Billion |
| Compound Annual Growth Rate | 39.1% |
| Regions Covered | Global |
| No. of Companies Mentioned | 23 |