The non-invasive prenatal testing market is expected to grow at a CAGR of 14.4% during the forecast period.
The COVID-19 pandemic impacted the non-invasive prenatal testing (NIPT) market due to the hold on non-invasive prenatal screening (NIPS) services to prevent the spread of the COVID-19 virus. The tremendous pressure on hospitals to provide services to COVID-19 patients was also one of the factors inhibiting hospitals and diagnostic centers from providing non-invasive prenatal screening (NIPS) services. According to the study published in the Journal of Clinical Medicine in March 2022, in Poland, the number of patients enrolled in the prenatal testing program fell compared to the previous year. In Poland, the number of integrated screening tests declined along with the number of newborns, while the percentage of triple tests increased. The number of invasive diagnostic tests also reduced significantly. Therefore, it is expected to negatively impact the market studied during the forecast period.
Factors propelling the growth of the market are the increasing number of babies with chromosomal disorders owing to the increasing number of late pregnancies, increasing demand for early and non-invasive fetal diagnosis, and favorable reimbursement policies. The increasing number of stillbirth cases across the world is also expected to offer a sizeable growth opportunity for early disease diagnosis. For instance, in April 2022, a research study published in the National Library of Medicine stated that approximately 0.4 to 0.9% of newborns have chromosomal abnormalities, and about half have an abnormal phenotype. According to the same source, approximately 1 in 5000 to 1 in 16000 live births have the third most common autosomal trisomy. Hence, with such cases of stillbirths, the demand for testing increases, which, in turn, is expected to aid the overall market growth during the forecast period.
Additionally, the advantages of non-invasive prenatal tests over other prenatal tests, such as their non-invasive nature, better accuracy, and short duration of the procedure, are anticipated to hasten the growth of the NIPT market during the forecast period. Furthermore, awareness programs by public organizations are another factor propelling the market growth. For instance, in April 2022, the US Food and Drug Administration alerted the public about the risk of misleading results, improper use, and inappropriate interpretation of results (NIPT).
However, the lack of skilled professionals, stringent regulations, and ethical concerns are expected to hinder market growth.
Non-invasive prenatal testing (NIPT) consists of analyzing cell-free DNA (cfDNA) circulating in the mother’s blood to detect Down syndrome. With the successful introduction of NIPT for detecting Down syndrome into routine prenatal care, it is important to understand the risks, benefits, and limitations to guide patients in making informed decisions. Non-invasive prenatal testing (NIPT) offers several clinical advantages over existing prenatal tests, such as maternal serum screening (MSS). According to the study by Stanislav Birko et al. published in January 2022, for the screening of Down syndrome, non-invasive prenatal testing (NIPT) can be performed as early as the ninth week of pregnancy, holds no risk of miscarriage, and can detect the presence of Down syndrome (trisomy 21) with high sensitivity (99.9%) and specificity (98%). As a result, the demand for these technologies has increased and is being adopted by many developing countries, which is expected to drive the growth of the segment.
Thus, the abovementioned factors are expected to increase market growth during the forecast period.
Furthermore, high diagnosis rates, wider adoption and awareness among the people, and the introduction of new and technologically advanced tests are also contributing to the market growth in North America. For instance, in May 2021, Yourgene launched IONA Care, a non-invasive prenatal test (NIPT) service offering to measure whether a pregnant woman is carrying a fetus with sex chromosome aneuploidies (SCA) and autosomal aneuploidies (AA), in addition to the current IONA test, which offers screening for trisomies 21, 18, and 13 and fetal sex determination. Thus, such product launches are expected to increase market growth over the forecast period.
Hence, the abovementioned factor will have a positive influence on the growth of the market studied during the forecasted period.
This product will be delivered within 2 business days.
The COVID-19 pandemic impacted the non-invasive prenatal testing (NIPT) market due to the hold on non-invasive prenatal screening (NIPS) services to prevent the spread of the COVID-19 virus. The tremendous pressure on hospitals to provide services to COVID-19 patients was also one of the factors inhibiting hospitals and diagnostic centers from providing non-invasive prenatal screening (NIPS) services. According to the study published in the Journal of Clinical Medicine in March 2022, in Poland, the number of patients enrolled in the prenatal testing program fell compared to the previous year. In Poland, the number of integrated screening tests declined along with the number of newborns, while the percentage of triple tests increased. The number of invasive diagnostic tests also reduced significantly. Therefore, it is expected to negatively impact the market studied during the forecast period.
Factors propelling the growth of the market are the increasing number of babies with chromosomal disorders owing to the increasing number of late pregnancies, increasing demand for early and non-invasive fetal diagnosis, and favorable reimbursement policies. The increasing number of stillbirth cases across the world is also expected to offer a sizeable growth opportunity for early disease diagnosis. For instance, in April 2022, a research study published in the National Library of Medicine stated that approximately 0.4 to 0.9% of newborns have chromosomal abnormalities, and about half have an abnormal phenotype. According to the same source, approximately 1 in 5000 to 1 in 16000 live births have the third most common autosomal trisomy. Hence, with such cases of stillbirths, the demand for testing increases, which, in turn, is expected to aid the overall market growth during the forecast period.
Additionally, the advantages of non-invasive prenatal tests over other prenatal tests, such as their non-invasive nature, better accuracy, and short duration of the procedure, are anticipated to hasten the growth of the NIPT market during the forecast period. Furthermore, awareness programs by public organizations are another factor propelling the market growth. For instance, in April 2022, the US Food and Drug Administration alerted the public about the risk of misleading results, improper use, and inappropriate interpretation of results (NIPT).
However, the lack of skilled professionals, stringent regulations, and ethical concerns are expected to hinder market growth.
Non-Invasive Prenatal Testing Market Trends
Down Syndrome Segment Dominates the Non-invasive Prenatal Testing Market
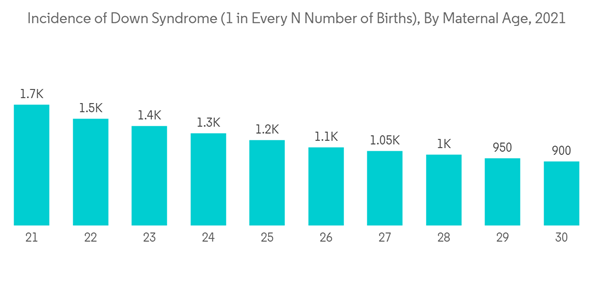
Down syndrome is a chromosomal disorder, also called trisomy 21, trisomy G, 47, XX,+21, and 47, XY,+21. It includes certain birth defects, learning problems, and deformities of facial features. A child with Down syndrome may also have heart defects and problems with vision and hearing. According to the Centers for Disease Control and Prevention data updated in April 2021, Down syndrome is the most common chromosomal condition diagnosed in the United States. Every year, about 6,000 babies born in the United States have Down syndrome. This means that Down syndrome occurs in about 1 out of every 700 babies. Hence, the growing incidence rate of down syndrome is expected to create lucrative opportunities for the growth of the market studied during the forecast period.Non-invasive prenatal testing (NIPT) consists of analyzing cell-free DNA (cfDNA) circulating in the mother’s blood to detect Down syndrome. With the successful introduction of NIPT for detecting Down syndrome into routine prenatal care, it is important to understand the risks, benefits, and limitations to guide patients in making informed decisions. Non-invasive prenatal testing (NIPT) offers several clinical advantages over existing prenatal tests, such as maternal serum screening (MSS). According to the study by Stanislav Birko et al. published in January 2022, for the screening of Down syndrome, non-invasive prenatal testing (NIPT) can be performed as early as the ninth week of pregnancy, holds no risk of miscarriage, and can detect the presence of Down syndrome (trisomy 21) with high sensitivity (99.9%) and specificity (98%). As a result, the demand for these technologies has increased and is being adopted by many developing countries, which is expected to drive the growth of the segment.
Thus, the abovementioned factors are expected to increase market growth during the forecast period.
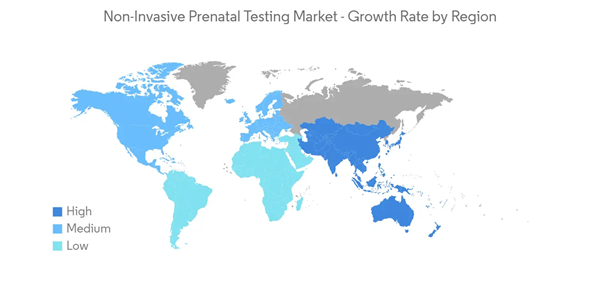
North America is Expected to Dominate the Market Over the Forecast Period
North America is expected to hold a leading position in the non-invasive prenatal testing (NIPT) market during the forecast period. High preterm birth rate, high maternal mortality rate, and rising incidences of chromosomal abnormalities will likely increase the adoption and demand for tests in this region. Various research studies provide insight into the effectiveness of awareness about prenatal care. For instance, in August 2022, a research study published in the National Library of Medicine stated that advances in antenatal care have resulted in increased awareness of fetal malformations, and malpresentation is expected to improve preparation for a high-risk delivery. Hence pregnant women need to adhere to prenatal care recommendations for optimal outcomes. Thus, North America is expected to dominate the non-invasive prenatal testing (NIPT) market over the forecast period due to the growing awareness of chromosomal abnormalities among fetuses.Furthermore, high diagnosis rates, wider adoption and awareness among the people, and the introduction of new and technologically advanced tests are also contributing to the market growth in North America. For instance, in May 2021, Yourgene launched IONA Care, a non-invasive prenatal test (NIPT) service offering to measure whether a pregnant woman is carrying a fetus with sex chromosome aneuploidies (SCA) and autosomal aneuploidies (AA), in addition to the current IONA test, which offers screening for trisomies 21, 18, and 13 and fetal sex determination. Thus, such product launches are expected to increase market growth over the forecast period.
Hence, the abovementioned factor will have a positive influence on the growth of the market studied during the forecasted period.
Non-Invasive Prenatal Testing Market Competitor Analysis
The non-invasive prenatal testing market is very competitive, with most players competing to maximize their market shares. Strong competition, rapid technological advancements, recurrent changes in government policies, and prices are key factors that confront the market. Key market players are BGI, Eurofins Scientific, F. Hoffmann-La Roche Ltd, Invitae Corporation, Illumina Inc., Natera Inc., Qiagen, Laboratory Corp. of America Holdings, and Quest Diagnostics, among others.Additional benefits of purchasing the report:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
This product will be delivered within 2 business days.
Table of Contents
1 INTRODUCTION
4 MARKET DYNAMICS
5 MARKET SEGMENTATION (Market Size by Value - USD million)
6 COMPETITIVE LANDSCAPE
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- BGI
- Eurofins Scientific
- F. Hoffmann-La Roche Ltd
- Invitae Corporation
- Illumina Inc.
- Natera Inc.
- PerkinElmer Inc.
- Centogene NV
- MedGenome Labs Ltd
- Myriad Womens Health Inc.
- Qiagen
Methodology

LOADING...