The mucosal atomization devices market is projected to record a CAGR of nearly 7.25% over the forecast period.
During the initial phase, the COVID-19 pandemic adversely affected communities, industries, and markets, including mucosal atomization devices. For instance, as per a survey conducted by the International Archives of Allergy and Immunology in January 2021, only 13% of physicians continued skin prick testing with commercial inhalant extracts, while 31% of physicians performed skin pricking tests in rare cases. However, all the respondents were observed to be continuing their asthma and allergic rhinitis medication, including inhaled corticosteroids, long-acting β2-agonists, Montelukast sodium, antihistamines, and nasal steroids. Over 92% of physicians reported prescribing inhaled medication to COVID-19 patients for instances of emergency management. In the current scenario, as the restrictions eased, the market is anticipated to witness growth because of the increase in the adoption of mucosal atomization devices due to the advantages offered by the devices in drug delivery. For instance, as per the article published in February 2022 in eBioMedicine journal, immune responses at the mucosal and systemic levels may be induced via mucosal immunization. Nine vaccines against different mucosal infections have been licensed for human use, including eight provided orally and one given intranasally (IN), all of which are complete viral vaccines.
The major factor contributing to the market growth is the increase in the prevalence of allergic rhinitis, sinus, and other target diseases across the world. For instance, as per the Allergy Capital Report 2022, one of the most common allergic conditions is seasonal allergic rhinitis, often called “hay fever.” About 7.7% of adults and 7.2% of children have been diagnosed with seasonal allergic rhinitis in the United States. Similarly, as per the June 2022 update from European Parliament, allergies are among the most common chronic non‑communicable diseases in Europe. In 2022, about 150 million people in Europe live with an allergic condition, such as allergic rhinitis, asthma, atopic eczema, or a food allergy. Hence, the rise in allergy diseases across the globe is anticipated to increase the demand for mucosal atomization devices, thereby driving market growth.
Mucosal atomization devices help in faster delivery of medication in the bloodstream, thus reducing the time for therapeutics to act on the concerned medical issue. As these drugs directly enter the bloodstream, they avoid first-pass metabolism and offer quicker results. Further, the increasing number of epilepsy cases among the population is increasing the adoption of mucosal atomization devices to quickly administer the drug intranasally in emergencies. For instance, according to an article published in Taylor and Francis, in March 2021, it had been observed that midazolam mucosal nasal spray rapidly absorbs, in adult and pediatric patients with seizures/epilepsy, and helps in terminating the seizure activity. This shows the viable tolerability of midazolam nasal spray through mucosal atomization devices in the patients which is expected to increase the demand for midazolam nasal spray for treating patients with epilepsy, thereby propelling the market growth.
Moreover, technological advancements and developments in mucosal atomization devices are expected to boost market growth since they increase convenience and thereby lead to the adoption of the devices. For instance, in October 2021, Alcove Manufacturing and Distribution Inc. and Pulmodyne Inc. collaborated to expand distribution, consolidate manufacturing, and continue cooperative research of novel atomization technologies. With this new partnership, Pulmodyne distributed the EZ-Spray Atomization System through its existing channels. In addition to distribution, the partnership seeks to innovate further in the space, and this relationship will allow the two experienced companies to optimize product designs quickly.
In the midst of these developments, side effects due to the overdose of drugs are expected to restrain the market growth.
Furthermore, due to the rapid rise in urbanization, industrialization, and the resultant lifestyle changes, a steep rise in air pollutants has been observed to increase the number of allergies caused by air pollution, such as asthma, allergic rhinitis, and others. For instance, according to the World Air Quality Report, published by IQAir, in March 2022, India is the fifth most polluted country among 117 countries, regions, and territories around the world. The annual average PM2.5 levels reached 58.1 micrograms per cubic meter (µg/m3) in 2021, in India. Thus, with the rising airborne allergens, the need for treatment is expected to increase which in turn is expected to promote market growth over the forecast period.
Moreover, the rise in technological advances and research and developments in gas-powered or propelled drug delivery systems are expected to boost segment growth. For instance, in January 2021, the FDA accepted Impel's 5O5 (b)(2) New Drug Application (NDA) for INP104, a dihydroergotamine mesylate (DHE) delivered directly into the vascular-rich upper nasal space using Impel’s proprietary Precision Olfactory Delivery (POD) technology, which is used for the acute treatment of migraine headaches with or without aura in adults.
Moreover, the rise in technological advancements and clinical development utilizing mucosal atomization devices are likely to drive market growth. For instance, as per the article published in January 2023 in PubMed, the researchers demonstrated that the M2-deficient single replication (M2SR) might provide substantial protection against infection with highly drifted strains of H3N2 influenza. The study utilized a mucosal atomization device (MAD301; Teleflex) for dose administration and prepared the final diluted product, which was drawn into 1-mL disposable polypropylene syringes for intranasal delivery.
This product will be delivered within 2 business days.
During the initial phase, the COVID-19 pandemic adversely affected communities, industries, and markets, including mucosal atomization devices. For instance, as per a survey conducted by the International Archives of Allergy and Immunology in January 2021, only 13% of physicians continued skin prick testing with commercial inhalant extracts, while 31% of physicians performed skin pricking tests in rare cases. However, all the respondents were observed to be continuing their asthma and allergic rhinitis medication, including inhaled corticosteroids, long-acting β2-agonists, Montelukast sodium, antihistamines, and nasal steroids. Over 92% of physicians reported prescribing inhaled medication to COVID-19 patients for instances of emergency management. In the current scenario, as the restrictions eased, the market is anticipated to witness growth because of the increase in the adoption of mucosal atomization devices due to the advantages offered by the devices in drug delivery. For instance, as per the article published in February 2022 in eBioMedicine journal, immune responses at the mucosal and systemic levels may be induced via mucosal immunization. Nine vaccines against different mucosal infections have been licensed for human use, including eight provided orally and one given intranasally (IN), all of which are complete viral vaccines.
The major factor contributing to the market growth is the increase in the prevalence of allergic rhinitis, sinus, and other target diseases across the world. For instance, as per the Allergy Capital Report 2022, one of the most common allergic conditions is seasonal allergic rhinitis, often called “hay fever.” About 7.7% of adults and 7.2% of children have been diagnosed with seasonal allergic rhinitis in the United States. Similarly, as per the June 2022 update from European Parliament, allergies are among the most common chronic non‑communicable diseases in Europe. In 2022, about 150 million people in Europe live with an allergic condition, such as allergic rhinitis, asthma, atopic eczema, or a food allergy. Hence, the rise in allergy diseases across the globe is anticipated to increase the demand for mucosal atomization devices, thereby driving market growth.
Mucosal atomization devices help in faster delivery of medication in the bloodstream, thus reducing the time for therapeutics to act on the concerned medical issue. As these drugs directly enter the bloodstream, they avoid first-pass metabolism and offer quicker results. Further, the increasing number of epilepsy cases among the population is increasing the adoption of mucosal atomization devices to quickly administer the drug intranasally in emergencies. For instance, according to an article published in Taylor and Francis, in March 2021, it had been observed that midazolam mucosal nasal spray rapidly absorbs, in adult and pediatric patients with seizures/epilepsy, and helps in terminating the seizure activity. This shows the viable tolerability of midazolam nasal spray through mucosal atomization devices in the patients which is expected to increase the demand for midazolam nasal spray for treating patients with epilepsy, thereby propelling the market growth.
Moreover, technological advancements and developments in mucosal atomization devices are expected to boost market growth since they increase convenience and thereby lead to the adoption of the devices. For instance, in October 2021, Alcove Manufacturing and Distribution Inc. and Pulmodyne Inc. collaborated to expand distribution, consolidate manufacturing, and continue cooperative research of novel atomization technologies. With this new partnership, Pulmodyne distributed the EZ-Spray Atomization System through its existing channels. In addition to distribution, the partnership seeks to innovate further in the space, and this relationship will allow the two experienced companies to optimize product designs quickly.
In the midst of these developments, side effects due to the overdose of drugs are expected to restrain the market growth.
Mucosal Atomization Devices Market Trends
Gas-propelled Mucosal Atomization Device is Expected to be a Fastest Growing Segment Over the Forecast Period
Gas-powered or propelled drug delivery systems were among the first developed systems. They use gas or an air cartridge attached to the gun either directly or indirectly through a tubing system to deliver power to the injector piston. When the trigger is activated, it releases the piston and creates a jet stream of medicant through the nasal and buccal passages and the trachea. Gas-propelled delivery offers the benefit of being extremely flexible. Owing to the variety of liquefied gases available, it’s possible to provide a complete spectrum of pressure ranges within a single container format. This allows a single-device system to manage a variety of delivery options, including different viscosities (up to 300 cP and much higher with a new variation on the technology), injection volumes, and primary containers.Furthermore, due to the rapid rise in urbanization, industrialization, and the resultant lifestyle changes, a steep rise in air pollutants has been observed to increase the number of allergies caused by air pollution, such as asthma, allergic rhinitis, and others. For instance, according to the World Air Quality Report, published by IQAir, in March 2022, India is the fifth most polluted country among 117 countries, regions, and territories around the world. The annual average PM2.5 levels reached 58.1 micrograms per cubic meter (µg/m3) in 2021, in India. Thus, with the rising airborne allergens, the need for treatment is expected to increase which in turn is expected to promote market growth over the forecast period.
Moreover, the rise in technological advances and research and developments in gas-powered or propelled drug delivery systems are expected to boost segment growth. For instance, in January 2021, the FDA accepted Impel's 5O5 (b)(2) New Drug Application (NDA) for INP104, a dihydroergotamine mesylate (DHE) delivered directly into the vascular-rich upper nasal space using Impel’s proprietary Precision Olfactory Delivery (POD) technology, which is used for the acute treatment of migraine headaches with or without aura in adults.
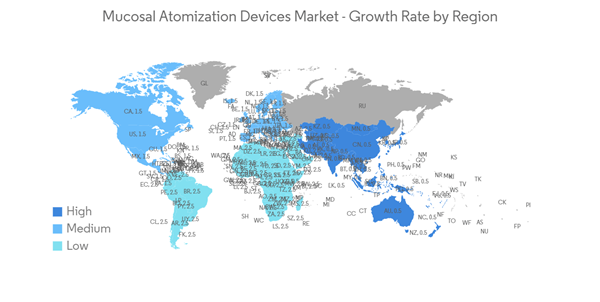
North America Anticipated to Hold a Significant Share in the Market Over the Forecast Period
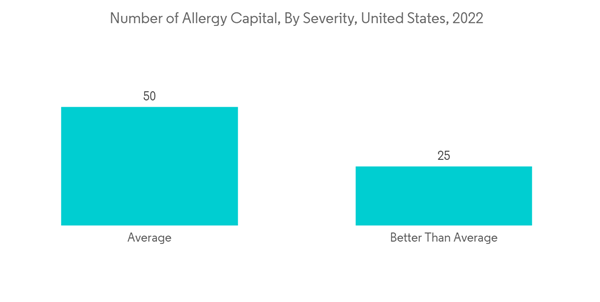
North America is expected to hold a significant market share in the global market throughout the forecast period due to factors such as the presence of key players, the high prevalence of allergic rhinitis and sinus in the region, and the established healthcare infrastructure. For instance, as per the AAFA allergy capital report 2022, Scranton in Pennsylvania ranked first for the worse-than-average allergy capital among others in the United States. Furthermore, as per the Asthma Canada 2021 report, every day, 317 Canadians are newly diagnosed with asthma. More than 850,000 children under the age of 14 have asthma, making it one of the most common chronic diseases among children in Canada. Hence, the rise in asthma and allergic disease cases increases the demand for mucosal atomization devices over the forecast period.Moreover, the rise in technological advancements and clinical development utilizing mucosal atomization devices are likely to drive market growth. For instance, as per the article published in January 2023 in PubMed, the researchers demonstrated that the M2-deficient single replication (M2SR) might provide substantial protection against infection with highly drifted strains of H3N2 influenza. The study utilized a mucosal atomization device (MAD301; Teleflex) for dose administration and prepared the final diluted product, which was drawn into 1-mL disposable polypropylene syringes for intranasal delivery.
Mucosal Atomization Devices Market Competitor Analysis
The mucosal atomization devices market is moderately competitive and consists of several players. Some of the companies currently dominating the market are Teleflex Incorporated, DeVilbiss Healthcare LLC, Becton, Dickinson and Company, Cook Group (Cook Medical), and Medica Holdings.Additional benefits of purchasing the report:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
This product will be delivered within 2 business days.
Table of Contents
1 INTRODUCTION
4 MARKET DYNAMICS
5 MARKET SEGMENTATION (Market Size by Value- USD Million)
6 COMPETITIVE LANDSCAPE
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Teleflex Incorporated
- DeVilbiss Healthcare LLC
- Becton, Dickinson and Company
- Cook Group
- Medica Holdings
- Integra LifeSciences
- BVM Meditech Private Limited
- Life-Assist Inc.
- BTME Group Limited (MEDTREE)