Speak directly to the analyst to clarify any post sales queries you may have.
Comprehensive introduction outlining how clinical priorities, technological convergence, and stakeholder expectations are reshaping veterinary orthopedic implants
The veterinary orthopedic implants sector is experiencing multifaceted evolution driven by clinical need, technological innovation, and changing stakeholder expectations. Clinicians are seeking implants and fixation systems that reduce operative time, improve healing trajectories, and lower complication rates for a diverse set of species. At the same time, pet owners and livestock managers increasingly expect evidence-based interventions that balance cost, recovery outcomes, and long-term functionality. This confluence of clinical priorities and owner expectations is reshaping product development priorities and supplier-contractor dynamics.
Technological convergence is also reshaping clinical practice. Advances in imaging, biomaterials, and manufacturing techniques are enabling implants that better match anatomical complexity across species, while surgical planning software and intraoperative guidance expand the capacity of veterinary surgeons to deliver consistent outcomes. Concurrently, regulatory scrutiny and the emphasis on post-market surveillance are increasing the evidentiary bar for new implants, prompting stronger clinical study programs and collaborative research between industry and academic centers.
As a result, strategic decisions must account for the interplay between clinical efficacy, manufacturability, and cost-effectiveness. Innovations that emphasize modularity, cross-species applicability, and streamlined sterilization or inventory management will capture clinician interest. Furthermore, sustained investment in clinician education and outcome tracking will accelerate adoption and support favorable reimbursement and procurement dialogues in both companion and large animal settings.
In-depth analysis of the transformative clinical, technological, and commercial shifts that are redefining the veterinary orthopedic implants landscape
The landscape of veterinary orthopedic implants has been reshaped by a set of transformative shifts that extend beyond incremental product updates to alter clinical pathways and commercial models. Material science breakthroughs have introduced bioabsorbable polymers and advanced coatings that improve osseointegration while reducing long-term foreign body burden. Concurrently, the broader adoption of additive manufacturing and precision machining has enabled more anatomically contoured plates and patient-adaptive implants, which reduce intraoperative modification and support faster rehabilitation.
Surgical innovation is also redefining standards of care. Minimally invasive approaches and enhanced fixation strategies allow for earlier weight-bearing and shorter hospitalization periods, which align with client preferences and operational efficiencies in veterinary hospitals and ambulatory centers. Digital workflows, including three-dimensional preoperative planning and intraoperative navigation, are becoming more accessible, thus narrowing the performance gap between specialist centers and general practitioners.
In parallel, supply chain resilience has emerged as a strategic priority. Vendors are diversifying sourcing and improving inventory visibility to mitigate disruption risk while also optimizing cost structures. Regulatory and reimbursement considerations have tightened, requiring more robust clinical evidence and traceability, which, in turn, favor companies that invest in clinical partnerships and post-market data collection. Taken together, these shifts are accelerating the move from commodity implants to differentiated systems that deliver demonstrable clinical value and operational advantages.
Rigorous assessment of how the 2025 tariff adjustments reshaped procurement, supply chain resilience, and strategic sourcing approaches across the implant ecosystem
The tariff measures enacted in 2025 introduced material implications for the veterinary orthopedic implants ecosystem by influencing cost structures, procurement behavior, and strategic sourcing. Rising duties on specific imported components and finished implants compelled suppliers and buyers to reassess the total landed cost of devices, which affected pricing discussions with clinics, hospitals, and large animal operations. In response, several manufacturers explored alternative material sourcing, adjusted component specifications where clinically appropriate, and accelerated supplier diversification to moderate exposure to tariff-driven price volatility.
Consequently, procurement teams adopted more rigorous cost-to-serve analyses and increased collaboration with suppliers to identify opportunities for value engineering without compromising clinical performance. Some organizations increased inventory buffers for critical components to reduce lead-time vulnerability, while others prioritized nearshoring or reshoring of key production steps to stabilize supply and preserve margin integrity. Additionally, the tariff environment incentivized investment in domestically manufacturable materials and modular designs that allowed parts substitution without extensive regulatory rework.
Clinicians and institutional purchasers experienced transitional friction as pricing adjustments and lead-time variability affected case planning and product availability. Nonetheless, these pressures also catalyzed innovation in product design and logistics, prompting a strategic shift toward resilient supply chains and deeper supplier partnerships. Going forward, the tariff-induced realignment underscores the importance of scenario planning and contractual flexibility to manage cost pressures while maintaining clinical continuity.
Key segmentation insights linking animal type, implant category, material composition, end-user setting, and clinical application to strategic product and service priorities
Segment-level dynamics reveal important nuances that inform product development, go-to-clinic strategies, and clinical training priorities. When analyzed by animal type, the landscape spans Exotic Animals, Large Animals, and Small Animals; Exotic Animals bifurcate into Birds and Reptiles, Large Animals into Bovine, Equine, and Porcine, and Small Animals into Cats and Dogs, each presenting distinct anatomical and biomechanical requirements that drive tailored fixation approaches. Implant type segmentation clarifies that external fixators exist as Ring Fixators and Unilateral Fixators, interlocking nails serve distinct long-bone stabilization roles, intramedullary nails differentiate into Cannulated Nails and Solid Nails, and plates and screws divide into DCP Plates and Locking Plates; these device subtypes reflect clinician preferences for construct stability, ease of insertion, and postoperative management.
Material type considerations further separate opportunities across Bioabsorbable Polymers, Ceramic Coatings, Stainless Steel, and Titanium, where biocompatibility, fatigue resistance, and cost all influence selection. End-user segmentation indicates that Ambulatory Surgical Centers, Research And Academic Institutes, Veterinary Clinics, and Veterinary Hospitals each demand different product features and service models, ranging from single-use simplicity to durable, high-performance systems supported by research-grade data. Application-based segmentation highlights fracture fixation, joint reconstruction, ligament repair, and osteotomy as procedural categories that determine implant geometry, instrumentation sets, and perioperative protocols.
Taken together, these segmentations reveal that successful products and strategies will be those that align implant design, material choice, and service delivery with the anatomical, clinical, and operational realities of each species, procedure type, and care setting.
Comprehensive regional insights highlighting how geographic differences in clinical practice, regulation, and supply chains influence product adoption and commercial strategy
Regional dynamics shape competitive positioning, regulatory navigation, and clinical adoption pathways across the veterinary orthopedic implants landscape. The Americas region exhibits concentrated demand from companion animal practices and integrated veterinary hospital networks, underpinned by strong clinical research institutions and established distribution channels; this environment favors solutions that combine clinical evidence, surgeon training, and scalable logistics. Europe, Middle East & Africa presents a heterogeneous regulatory and reimbursement environment where high-volume centers in specific countries drive innovation adoption while other jurisdictions emphasize cost containment and local procurement, making regulatory strategy and local partnerships essential for market entry and expansion. Asia-Pacific demonstrates a rapid cadence of clinical capability building and manufacturing scale, with significant growth in specialized surgical centers and an expanding base of trained veterinary orthopedic surgeons, creating opportunities for regionally adapted product designs and manufacturing collaborations.
Across these geographies, differences in companion animal ownership trends, livestock industry structures, and public animal health priorities influence demand for specific implant types and materials. Variations in regulatory timelines and import policies also affect go-to-market sequencing and the appropriateness of near-market clinical studies. Therefore, a differentiated regional strategy that aligns regulatory engagement, clinical education, and supply chain design with local practice patterns will be central to sustainable adoption and long-term commercial success.
Strategic company-level insights into competitive differentiation driven by clinical partnerships, manufacturing quality, and integrated digital and training capabilities
Competitive dynamics in the veterinary orthopedic implants space reflect a balance between established device manufacturers, specialized orthopedic suppliers, and agile niche entrants. Market leaders tend to differentiate through integrated portfolios that combine instrumentation, implants, and training programs, while emerging players focus on material innovation, modular systems, or species-specific solutions to carve distinct clinical niches. Strategic partnerships between device firms and academic or clinical centers are increasingly common, enabling rapid evidence generation and facilitating surgeon endorsement for novel technologies.
Manufacturing excellence and quality systems remain pivotal, with suppliers that demonstrate consistent device performance, reliable sterilization and packaging, and robust traceability gaining procurement preference. At the same time, companies that invest in digital tools-such as surgical planning software, outcome tracking platforms, and tele-education-stand to strengthen clinical adoption and generate post-market evidence. Mergers and strategic collaborations are reshaping competitive boundaries, as larger firms integrate niche technologies to broaden clinical coverage and as smaller companies seek distribution partnerships to scale reach.
Operational agility, including flexible manufacturing contracts and responsive customer service, differentiates top-performing suppliers. Moreover, transparent clinical data and sound post-market surveillance programs will increasingly inform purchasing committees and institutional formularies, elevating the importance of coordinated clinical engagement and outcomes measurement in commercial strategies.
Actionable strategic recommendations for industry leaders to align product innovation, supply chain resilience, and clinical engagement for sustainable growth
Practical recommendations for industry leaders focus on aligning product innovation with clinical utility, strengthening supply resilience, and accelerating clinician engagement. Prioritize development of implant systems that reduce intraoperative complexity and support expedited rehabilitation, because usability improvements translate directly into broader clinical adoption and improved patient outcomes. Simultaneously, invest in materials and designs that minimize inventory complexity by offering modularity across species and procedures, thereby enhancing distribution efficiency and lowering lifecycle costs for end users.
Strengthen supply chain resilience by diversifying suppliers, qualifying secondary sources for critical components, and evaluating nearshoring options where feasible to reduce exposure to trade actions and logistical bottlenecks. In parallel, establish outcome-tracking collaborations with veterinary hospitals and surgical centers to build robust clinical evidence that supports product differentiation and informs iterative design improvements. Forge targeted educational programs and hands-on training to accelerate surgeon proficiency with new systems, and couple these initiatives with digital decision-support tools that facilitate preoperative planning and postoperative monitoring.
Finally, engage proactively with regional regulators and procurement stakeholders to anticipate compliance requirements and position products for smoother market entry. By integrating these actions into a coordinated go-to-market plan, industry leaders can improve clinical impact, mitigate operational risks, and secure long-term value capture.
Transparent mixed-methods research methodology combining expert interviews, clinical literature review, and data triangulation to validate practical and technical insights
This research employed a mixed-methods approach combining primary qualitative engagement with domain experts and secondary review of clinical, regulatory, and technical literature to ensure a comprehensive understanding of trends and practical implications. Primary workstreams included structured interviews with practicing veterinary surgeons across companion and large animal specialties, procurement professionals at veterinary hospitals and ambulatory centers, product development leaders at implant manufacturers, and supply chain managers involved in device logistics. These interviews provided direct insight into clinical requirements, procedural preferences, and procurement decision criteria.
Secondary research incorporated peer-reviewed surgical literature, regulatory guidance documents, device labeling and technical specifications, and case studies related to implant performance and clinical outcomes. Data triangulation techniques were applied to reconcile divergent viewpoints between clinical practice and supplier positioning, and iterative validation sessions with subject-matter experts refined the interpretation of findings. Where appropriate, bench testing standards and material performance attributes were reviewed to contextualize design recommendations.
Methodological limitations include potential regional variation in clinical practice that may not be fully captured in the sample, and the inherent lag between emerging clinical innovations and published outcome data. To mitigate these constraints, the study emphasizes cross-validated clinical input and continuous stakeholder engagement to ensure practical relevance and evidentiary robustness.
Conclusive synthesis emphasizing the strategic imperative for integrated clinical evidence, resilient operations, and segmentation-driven product development
In conclusion, the veterinary orthopedic implants domain is transitioning from commodity-driven procurement toward clinically differentiated solutions that deliver demonstrable outcomes across species and care settings. Advances in materials, manufacturing, and digital planning are enabling more anatomically precise and functionally resilient implants, while regulatory and tariff pressures are reshaping procurement and supply strategies. Consequently, suppliers that pair clinical evidence with operational reliability and targeted training will capture clinician trust and institutional preference.
Strategically, the imperative is to design implants and service models that address the full continuum of clinical need-from emergency fracture fixation to complex joint reconstruction-while maintaining supply chain flexibility and regulatory preparedness. Regional differences in clinical capacity and procurement models necessitate adaptive market approaches, and segmentation-driven product planning will ensure that offerings match the procedural and species-specific realities clinicians face. Ultimately, organizations that integrate product innovation with robust clinical partnerships and resilient operations will be best positioned to deliver both clinical impact and sustainable commercial performance.
Additional Product Information:
- Purchase of this report includes 1 year online access with quarterly updates.
- This report can be updated on request. Please contact our Customer Experience team using the Ask a Question widget on our website.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
17. China Veterinary Orthopedic Implants Market
Companies Mentioned
The key companies profiled in this Veterinary Orthopedic Implants market report include:- Arthrex, Inc.
- B. Braun Melsungen AG
- BioMedtrix, Inc.
- DePuy Synthes, Inc.
- IMEX Veterinary Inc.
- Kyon AG
- Orthomed, Inc.
- Smith & Nephew plc
- Stryker Corporation
- Zimmer Biomet Holdings, Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 188 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
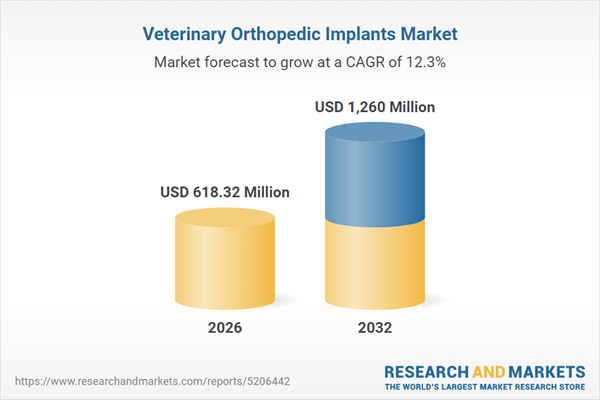
| Estimated Market Value ( USD | $ 618.32 Million |
| Forecasted Market Value ( USD | $ 1260 Million |
| Compound Annual Growth Rate | 12.3% |
| Regions Covered | Global |
| No. of Companies Mentioned | 11 |