Speak directly to the analyst to clarify any post sales queries you may have.
Funding Support in Europe for CGTs: US companies have primarily driven the growth of investments in CGT projects in recent years. The US market saw a 53% increase in CGT investment in 2021 compared to the previous year, while Europe experienced an 8% loss of funding. For European CGT development to keep up with global trends, additional funds are required to compensate for the loss in investment from 2021. More collaboration and investment from the European pharmaceutical market into academic research projects in CGTs are needed. Governments, including CGTs, can drive such collaborations and further investment to increase their position in the biopharma market. These initiatives can affect more private investment and talent in Europe, creating conditions needed to turn the European CGT market around.
Rising use of CGT Products for Disease Care: Cell & gene therapy solutions treat various diseases. They help to diagnose diseases by replacing, inactivating, and introducing genes into the body. Cell & gene therapy has a different way of treating diseases. Some of the biggest strides in cell & gene therapy are in the field of oncology, leading to targeted, personalized treatments that positively influence the market growth. Different technologies and customized treatments have driven the demand for cell & gene therapy solutions over the last few decades. In disease diagnosis, cell therapies are mainly used for cancer, diabetes, genetic disorders, musculoskeletal diseases, eye disorders, and other diseases. As per the Medicine in Development report, there are around 250+ products in the development stage for a broad range of diseases.
EUROPE CELL AND GENE THERAPY MARKET INSIGHTS
- By product, in 2023, the gene therapy segment occupied the largest share of the Europe cell and gene therapy market. The growing research in gene therapy techniques and development is expected to boost market growth. The increasing application of gene therapies in disease diagnosis, rising funding for gene therapies in small and medium-sized manufacturing companies, and the rapid increase in new drug applications will create new market space in the upcoming years.
- The oncology application segment showcases significant growth, with the fastest-growing CAGR of over 38% during the forecast period. The high success rate of cell and gene therapy solutions against cancer, the high application rate of cell and gene therapy products on various cancers, and the high investment and rapidly increasing development in oncology regenerative medicines drive the growth of this segment.
- The hospital end-user segment. The increasing collaboration and partnerships between manufacturers and hospitals, the shift from traditional hospital environments to gene therapy production, the growing demand for advanced therapy medicine products over traditional treatments, and revenue generation opportunities for health systems, including in-house cell and gene therapy manufacturing, are major factors fueling the growth of the hospital segment in the cell and gene therapy market.
SEGMENTATION & FORECAST BY
- Product
- Gene Therapy
- Cell Therapy
- Application
- Oncology
- Genetic Disorders
- Dermatology
- Musculoskeletal
- Others
- End-user
- Hospitals
- Cancer Care Centers
- Others
REGIONAL ANALYSIS
Germany held the largest share, over 21%, in the European cell and gene therapy market in 2023. Germany is at the forefront of cell and gene therapy due to major investments by the public and private sectors, which have boosted the development of the country's cell and gene therapy market. Germany is actively developing and testing innovative cell and gene therapy medicines, which offer patients vast benefits.- Region
- Germany
- France
- UK
- Spain
- Italy
- Rest of Europe
VENDOR LANDSCAPE
The Europe cell and gene therapy market report includes exclusive data on 36 vendors. The Europe cell & gene therapy market is highly competitive, driven by the increasing number of biopharmaceutical companies developing new therapies. Moreover, the rising financial support and government initiatives for regenerative medicines and advanced medicinal products boost key players in the market. Leading market players in the Europe CGT market are Novartis, Gilead Sciences, Amgen, F. Hoffmann-La Roche, Orchard Therapeutics, BioMarin Pharmaceutical, PTC Therapeutics, Bristol-Myers Squibb, Organogenesis, Smith & Nephew, Legend Biotech, CSL Ltd, Vertex Pharmaceuticals Inc, and Human Stem Cells Institute PJSC, which are competing with rapidly emerging market players in the market. Vendors in the market are increasing their market share through inorganic growth.Key Vendors
- Novartis
- Gilead Sciences
- Amgen
- F. Hoffmann-La Roche
- Orchard Therapeutics
- BioMarin Pharmaceutical
- PTC Therapeutics
- Bristol-Myers Squibb
- Organogenesis
- Smith & Nephew
- Legend Biotech
- CSL Ltd
- Vertex Pharmaceuticals Inc
- Human Stem Cells Institute PJSC
Other Prominent Vendors
- CHIESI Farmaceutici
- CollPlant
- CO.DON
- Takeda Pharmaceutical Company
- Nipro
- Vericel
- Avita Medical
- GenSight
- AGC Biologics
- Atara Biotherapeutics Inc
- NuVasive
Key Emerging Vendors
- Adaptimmune Therapeutics
- AgenTus Therapeutics
- Autolus Therapeutics
- Cellectis
- Celyad
- CombiGene AB
- Eukarys
- FREELINE Therapeutics
- Innoskel
- Psioxus Therapeutics
- Sparing Vision
KEY QUESTIONS ANSWERED
1. Who are the major Europe cell and gene therapy market players?2. Which region dominates the Europe cell and gene therapy market?
3. What are the Europe cell and gene therapy market trends?
4. What is the growth rate of the Europe cell and gene therapy market?
5. How big is the Europe cell and gene therapy market?
Table of Contents
Companies Mentioned
- Novartis
- Gilead Sciences
- Amgen
- F. Hoffmann-La Roche
- Orchard Therapeutics
- BioMarin Pharmaceutical
- PTC Therapeutics
- Bristol-Myers Squibb
- Organogenesis
- Smith & Nephew
- Legend Biotech
- CSL Ltd
- Vertex Pharmaceuticals Inc
- Human Stem Cells Institute PJSC
- CHIESI Farmaceutici
- CollPlant
- CO.DON
- Takeda Pharmaceutical Company
- Nipro
- Vericel
- Avita Medical
- GenSight
- AGC Biologics
- Atara Biotherapeutics Inc
- NuVasive
- Adaptimmune Therapeutics
- AgenTus Therapeutics
- Autolus Therapeutics
- Cellectis
- Celyad
- CombiGene AB
- Eukarys
- FREELINE Therapeutics
- Innoskel
- Psioxus Therapeutics
- Sparing Vision
Methodology
Our research comprises a mix of primary and secondary research. The secondary research sources that are typically referred to include, but are not limited to, company websites, annual reports, financial reports, company pipeline charts, broker reports, investor presentations and SEC filings, journals and conferences, internal proprietary databases, news articles, press releases, and webcasts specific to the companies operating in any given market.
Primary research involves email interactions with the industry participants across major geographies. The participants who typically take part in such a process include, but are not limited to, CEOs, VPs, business development managers, market intelligence managers, and national sales managers. We primarily rely on internal research work and internal databases that we have populated over the years. We cross-verify our secondary research findings with the primary respondents participating in the study.

LOADING...
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 103 |
| Published | June 2024 |
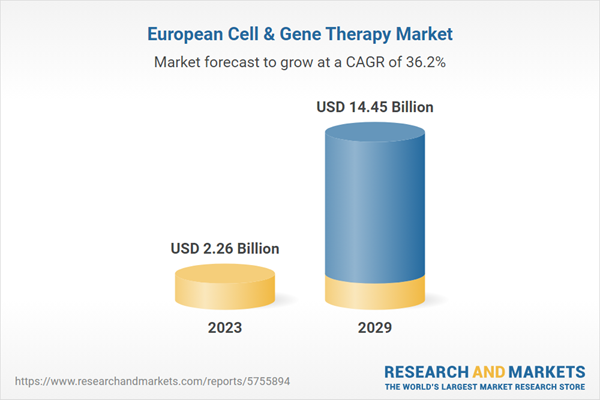
| Forecast Period | 2023 - 2029 |
| Estimated Market Value ( USD | $ 2.26 Billion |
| Forecasted Market Value ( USD | $ 14.45 Billion |
| Compound Annual Growth Rate | 36.2% |
| Regions Covered | Europe |
| No. of Companies Mentioned | 36 |