Global Breakthrough Therapy (BT) Designation Market - Key Trends & Drivers Summarized
How Is Breakthrough Therapy Designation Accelerating Drug Development?
The Breakthrough Therapy (BT) designation is transforming the drug development landscape by providing a fast-track approval process for therapies that show significant potential in treating serious or life-threatening conditions. Introduced by the U.S. Food and Drug Administration (FDA) in 2012, the BT designation is designed to expedite the development and review of new drugs that demonstrate substantial improvement over existing therapies in early clinical trials. This designation provides sponsors with more frequent FDA communication, priority review, and access to senior FDA officials, all aimed at accelerating the drug approval process and bringing innovative treatments to patients more quickly.BT designation is particularly beneficial for therapies targeting unmet medical needs, such as rare diseases, cancers, and chronic conditions that currently lack effective treatment options. For example, several immunotherapies and targeted therapies for cancer have received BT designation due to their ability to achieve higher response rates and improve patient outcomes compared to standard treatments. By shortening the time between clinical development and market approval, BT designation allows patients with serious conditions to access life-saving or life-altering therapies sooner, while also encouraging pharmaceutical companies to invest in groundbreaking research.
What Role Does Breakthrough Therapy Designation Play in Treating Rare Diseases?
Breakthrough Therapy (BT) designation plays a crucial role in the development and approval of treatments for rare diseases, which often lack effective therapies due to the small patient populations and the complexity of these conditions. Rare diseases, also known as orphan diseases, affect fewer than 200,000 people in the U.S., making drug development challenging due to limited financial incentives and difficulties in conducting large clinical trials. The BT designation helps overcome these barriers by providing a more efficient regulatory pathway for promising therapies, enabling faster development timelines and reducing the cost and risk associated with bringing new treatments to market.For rare disease therapies, the BT designation is often granted based on early clinical trial data showing significant efficacy in addressing the disease's underlying causes or symptoms. This expedited pathway is critical for patients with rare diseases, many of whom face limited treatment options or rapidly progressing conditions. By providing enhanced regulatory support and guidance, the BT designation helps ensure that promising therapies for rare diseases reach patients as quickly as possible. The rise of gene therapies, enzyme replacement therapies, and precision medicine approaches targeting rare genetic disorders has further highlighted the importance of the BT designation in accelerating the approval of innovative treatments for rare diseases.
How Is Breakthrough Therapy Designation Impacting Oncology Drug Development?
Breakthrough Therapy (BT) designation has had a profound impact on oncology drug development by accelerating the approval of novel cancer therapies that offer significant benefits over existing treatments. Cancer remains one of the leading causes of death globally, and the development of new, more effective therapies is critical to improving patient survival rates. BT designation is frequently awarded to oncology drugs that show strong efficacy in early clinical trials, particularly in terms of tumor shrinkage, progression-free survival, and overall survival rates. These promising results often come from immunotherapies, targeted therapies, and personalized medicine approaches that target specific genetic mutations or immune pathways in cancer cells.The BT designation has been instrumental in fast-tracking the approval of several groundbreaking cancer therapies, including immune checkpoint inhibitors and CAR-T cell therapies, which have shown remarkable success in treating certain types of cancers that were previously difficult to manage. For example, therapies targeting PD-1/PD-L1 pathways in cancers such as melanoma, lung cancer, and kidney cancer have received BT designation due to their ability to deliver improved clinical outcomes compared to traditional chemotherapy or radiation. By providing oncology drug developers with more frequent interactions with the FDA, BT designation ensures that these innovative therapies can navigate the regulatory process more efficiently and reach patients sooner, ultimately improving cancer care.
What Factors Are Driving the Growth of Breakthrough Therapy (BT) Designation?
The growth of the Breakthrough Therapy (BT) designation market is driven by several key factors, including the increasing focus on developing therapies for rare diseases, the growing demand for personalized medicine, and the rise of innovative treatment modalities such as gene therapy and immunotherapy. As pharmaceutical companies and biotech firms invest in the discovery of novel treatments for serious and life-threatening conditions, BT designation offers an attractive pathway for bringing these therapies to market more quickly. The rising prevalence of cancers, rare genetic disorders, and chronic diseases is also contributing to the demand for BT designation, as patients and healthcare providers seek more effective treatment options.The success of previously approved BT-designated drugs, particularly in oncology and rare disease treatment, has encouraged more pharmaceutical companies to pursue this expedited approval pathway. Additionally, advancements in genetic testing, molecular diagnostics, and biomarker identification are driving the development of personalized therapies that can target specific disease mechanisms more effectively. These therapies are often eligible for BT designation due to their potential to deliver significant clinical benefits. As precision medicine and biotechnology continue to advance, the BT designation market is expected to grow, providing regulatory support for a wide range of innovative therapies aimed at addressing unmet medical needs.
Report Scope
The report analyzes the Breakthrough Therapy (BT) Designation market, presented in terms of market value (US$ Thousand). The analysis covers the key segments and geographic regions outlined below.- Segments: Application (Oncology, Infectious Diseases, Rare Diseases, Pulmonary Diseases, Other Applications).
- Geographic Regions/Countries:World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; and Rest of Europe); Asia-Pacific; Rest of World.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Oncology Application segment, which is expected to reach US$138.3 Billion by 2030 with a CAGR of a 16.5%. The Infectious Diseases Application segment is also set to grow at 13.7% CAGR over the analysis period.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Breakthrough Therapy (BT) Designation Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Breakthrough Therapy (BT) Designation Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Breakthrough Therapy (BT) Designation Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Abbvie, Acadia, Alexion, Amgen, AstraZeneca and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 42 companies featured in this Breakthrough Therapy (BT) Designation market report include:
- Abbvie
- Acadia
- Alexion
- Amgen
- AstraZeneca
- BMS
- Boehringer Ingelheim
- Eisai
- Eli Lilly
- Exelixis
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Abbvie
- Acadia
- Alexion
- Amgen
- AstraZeneca
- BMS
- Boehringer Ingelheim
- Eisai
- Eli Lilly
- Exelixis
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 135 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
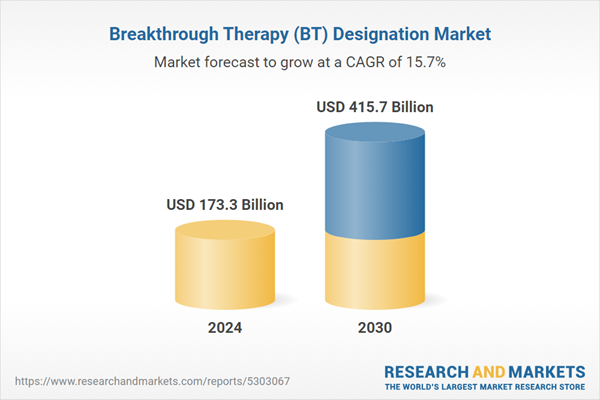
| Estimated Market Value ( USD | $ 173.3 Billion |
| Forecasted Market Value ( USD | $ 415.7 Billion |
| Compound Annual Growth Rate | 15.7% |
| Regions Covered | Global |