Speak directly to the analyst to clarify any post sales queries you may have.
MARKET TRENDS & OPPORTUNITIES
Advances and Integration of Technologies in Clinical Lab Testing
Clinical labs have seen significant advances due to enhanced computer technology. The days of manually performing lab tests have finally been replaced by exact, compact lab instruments offering rapid throughput - the rapidly increasing interest in the total establishment of automated labs noticed in the COVID-19 pandemic. In recent years, the U.S. clinical laboratory tests market has become more advanced by integrating new technologies, such as robotics, AI, Machine Learning, Cloud computing, and other tools for patient data management. One of the leading technology integrations is AI. It is a transformative element in clinical laboratories. It can scour large data sets to understand and identify the patterns and unknown linkage between disease, environmental conditions, and hereditary factors. These innovations led to early detection and public health and advanced personalized medicine.Telemedicine And Direct-To-Consumer Solutions Will Be the New Opportunities for Clinical Laboratories
The COVID-19 pandemic tremendously transformed clinical laboratory operations. In the wake of the COVID crisis, deep-seated cultural changes were noticed across the US and global clinical labs, and home testing became universal. Patients also expect clinical lab diagnostic testing to shift from traditional collection points (provider's offices, hospitals, and others) to homes and pharmacies.Hospitals are Integrating with Clinical Laboratories
With the rising demand for clinical testing, hospitals are experiencing a huge burden in the United States, and integrating hospitals with laboratories or established laboratories is an emerging trend. Hospitals are better positioned to deliver the best possible care with advanced laboratories. From healthcare systems in major cities to community hospitals/clinics across the country, every healthcare setting demands lab tests while maintaining the accuracy, quality, and effective result delivery for medical professionals to make timely decisions about patient’s health. Integrated laboratories with hospitals are an efficient part of rapid diagnosis. For instance, LabCorp is managing Ascension Hospitals Labs in around ten states of the US. The company offers a broad range of partnership models to healthcare systems and medical professionals to enhance lab services access across the United States.Increasing Utilization of Clinical Lab Tests
In the U.S., around 14 billion clinical lab tests are performed annually, making them the most used medical benefits. Across the country, more than 75% of clinical decisions are dependent on clinical lab tests. Over the past few years, the demand for clinical lab tests and services has been reflected in increased lab test expenditure nationwide. The Medicare program spent around USD 10 billion on clinical lab tests and witnessed a 17% increase from 2020 to 2021 in the US.High Demand for Early & Preventive Diagnosis
The awareness of early disease diagnosis and disease prevention in the U.S. is significant compared to the global population. Billions of clinical lab tests are performed annually across the country, which is significantly higher than in other countries. The culture of America (the U.S.) medicine has long supported and believed that early detection, treatment, and prevention are best.SEGMENTATION INSIGHTS
INSIGHTS BY TEST COMPLEXITY
The specialty test segment is the dominant test complexity segment in the U.S. clinical laboratory tests market 2023. Specialty tests are performed less than routine tests, but the price of specialty tests plays a vital role in segmental revenue growth. In clinical laboratories, more than 30% of lab tests are specialty lab tests. In addition, the increasing support by the government in specialty test development and delivery and insurance coverage accelerates the segmental growth. Furthermore, specialty tests are complex and segmented into moderately complex and highly complex testing. In specialty testing, genetics testing is a rapidly growing sector where the specialty testing segment obtains high revenue growth.Segmentation by Test Complexity
- Specialty Tests
- Routine Tests
INSIGHTS BY PROVIDERS
The U.S. clinical laboratory tests market by providers is segmented into hospital-based clinical laboratories, independent laboratories, physician office laboratories, and others. The hospital-based laboratories segment accounted for the largest U.S. clinical laboratory tests market share in 2023. In the healthcare sector, hospital-associated laboratories are at the forefront for patients. Hospital settings are the primary contributor to clinical lab services with the potential to deliver lab tests and diagnostics for outpatients and in-patient requirements, as well as walk-in consumers looking to benefit from the facilities' clinical expertise after receiving the results. Further, hospital-based laboratories conduct significant clinical lab tests and commonly perform more tests than other laboratories. There is one trend that hospitals are integrating with medical laboratories in the United States. The majority of hospitals are in demand for integrated labs. Quest Diagnostics and LabCorp are the two leading clinical labs witnessing increasing hospital demand for integration. These emerging trends are expected to deliver revenue growth opportunities.Segmentation by Providers
- Hospital-based Clinical Laboratory
- Independent Laboratories
- Physician Office Laboratories (PLOS)
- Others
INSIGHTS BY TEST TYPE
The U.S. clinical laboratory tests market by test type is segmented into clinical & immunochemistry, molecular diagnostics, hematology, microbiology & cytology, and toxicology. Clinical and immunochemistry types of clinical lab tests account for a higher market share and dominate other tests. Identifying biomolecules in human specimens in clinical chemistry tests describes health conditions and predicts future disease conditions. In clinical chemistry, the instrumental analysis of blood components. The major tests are enzymology, toxicology, and endocrinology, specialty tests that account for the segment's high revenue growth. In addition, immunochemistry testing is a highly demanding clinical lab test in the US. These tests are performed to identify the antibodies, detect the immune health issue, and determine the organ, tissue, and fluid compatibility during organ transplantation procedures.Segmentation by Test Complexity
- Specialty Tests
- Routine Tests
VENDOR LANDSCAPE
The U.S. clinical laboratory tests market is highly competitive, with a wide range of hospital-based laboratories, POLs, and independent clinical and anatomical pathology laboratories. The market is intensely competitive, and consolidation will likely continue in the United States. In addition, an increasing number of health system laboratories are expanding their operations and businesses, resulting in greater competition for clinical laboratory testing from physicians within those health systems and unaffiliated physicians in the health system laboratories’ service areas. Further, most U.S. clinical laboratory test market companies offer routine or specialized clinical diagnostic testing services or both depending on their scientific expertise, technologies, innovations, and relevant regulations. Based on that, the competitive landscape in the country for the clinical laboratory test market is highly localized and challenging.Key Company Profiles
- Eurofins Scientific
- Laboratory Corporation of America
- Mayo Clinic Laboratories
- OPKO Health, Inc. (BioReference Health)
- Quest Diagnostics
- Siemens Healthineers
- Sonic Healthcare
Other Prominent Vendors
- Accu Reference Medical Labs
- ARUP Laboratories
- Clinical Reference Laboratory
- DaVita
- Empire City Laboratories
- Fresenius Medical Care
- HealthTrackRx
- Laboratory of Florida
- Millenium Health
- US Speciality Labs
KEY QUESTIONS ANSWERED:
1. How big is the U.S. clinical laboratory tests market?2. What is the growth rate of the U.S. clinical laboratory tests market?
3. Which provider segment dominates the U.S. clinical laboratory tests market share?
4. What are the significant U.S. clinical laboratory test market trends?
5. Who are the U.S. clinical laboratory test market's key players?
Table of Contents
Companies Mentioned
- Eurofins Scientific
- Laboratory Corporation of America
- Mayo Clinic Laboratories
- OPKO Health, Inc. (BioReference Health)
- Quest Diagnostics
- Siemens Healthineers
- Sonic Healthcare
- Accu Reference Medical Labs
- ARUP Laboratories
- Clinical Reference Laboratory
- DaVita
- Empire City Laboratories
- Fresenius Medical Care
- HealthTrackRx
- Laboratory of Florida
- Millenium Health
- US Speciality Labs
Methodology
Our research comprises a mix of primary and secondary research. The secondary research sources that are typically referred to include, but are not limited to, company websites, annual reports, financial reports, company pipeline charts, broker reports, investor presentations and SEC filings, journals and conferences, internal proprietary databases, news articles, press releases, and webcasts specific to the companies operating in any given market.
Primary research involves email interactions with the industry participants across major geographies. The participants who typically take part in such a process include, but are not limited to, CEOs, VPs, business development managers, market intelligence managers, and national sales managers. We primarily rely on internal research work and internal databases that we have populated over the years. We cross-verify our secondary research findings with the primary respondents participating in the study.

LOADING...
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 186 |
| Published | February 2024 |
| Forecast Period | 2023 - 2029 |
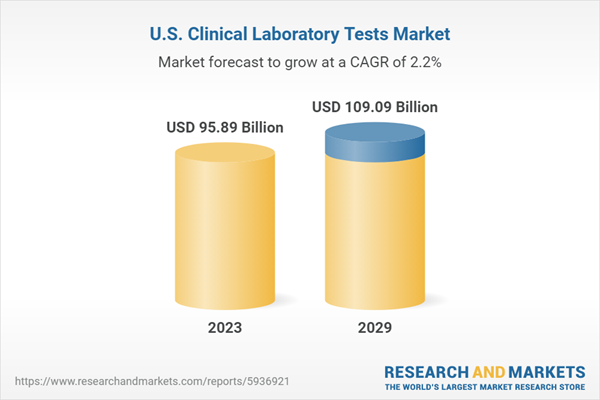
| Estimated Market Value ( USD | $ 95.89 Billion |
| Forecasted Market Value ( USD | $ 109.09 Billion |
| Compound Annual Growth Rate | 2.1% |
| Regions Covered | United States |
| No. of Companies Mentioned | 17 |