Speak directly to the analyst to clarify any post sales queries you may have.
Introduction to the evolving aneurysm coiling and embolization device landscape and the critical clinical and market forces reshaping therapy pathways
Aneurysm management continues to be a focal point of neurovascular care as clinicians balance the twin imperatives of effective occlusion and preservation of parent vessel integrity. Recent device innovations, procedural refinements, and evolving clinical guidelines have converged to expand the therapeutic toolbox for intracranial aneurysms. This executive summary synthesizes the clinical, regulatory, and commercial dynamics that are shaping practice patterns and influencing stakeholder decisions across the care continuum.
Clinicians increasingly select from a spectrum of device options based on aneurysm morphology, rupture risk, and patient-specific factors. These therapeutic options include traditional coils and more recent classes such as flow diverters and liquid embolics, each bringing distinct procedural workflows and evidence considerations. In parallel, procedural settings range from ambulatory surgical centers to tertiary hospitals and specialty clinics, which affects throughput, case mix, and peri-procedural support capabilities.
As reimbursement frameworks and procurement strategies evolve, manufacturers and providers must move beyond product features to align evidence generation, training, and distribution models with institutional priorities. This introduction frames the subsequent sections by highlighting trends in device design, clinical adoption, and supply chain considerations that together determine how aneurysm coiling and embolization devices will be used in contemporary neurovascular practice. The goal is to provide clarity for decision-makers seeking to translate technological advances into improved patient outcomes and sustainable commercial models.
Transformative shifts driving neurovascular intervention practice including device innovation, clinical adoption patterns, reimbursement evolution and procedural preferences
Over the past several years, the neurovascular sector has undergone transformative shifts driven by technological innovation, expanding clinical evidence, and changes in procedural environments. Device evolution has been marked by improvements in coil materials and coatings, refinements in flow diversion designs that prioritize metal coverage and flexibility, and the maturation of liquid embolic formulations that offer improved control and radiopacity. These technological advances have enabled interventionalists to approach a broader range of aneurysm anatomies with heightened confidence, and they have catalyzed procedural preference changes in many centers.
Clinical adoption has been influenced by the increasing availability of comparative outcomes data and registry information that clarify relative safety and efficacy across device classes. As a result, decision-making has shifted from a purely device-centric focus to a more nuanced assessment that integrates procedural complexity, operator experience, and peri-procedural resource requirements. Concurrently, training models are evolving to include simulation, proctoring programs, and modular curricula that accelerate operator proficiency while reducing variability in outcomes.
From a commercial perspective, companies are pursuing differentiated pathways by combining product innovation with services such as clinical support, reimbursement assistance, and curated training. Reimbursement policies and hospital procurement priorities are increasingly attentive to total procedure cost, length of stay, and long-term outcomes, which influences purchasing decisions and preference for devices that demonstrably reduce retreatment or complication rates. Taken together, these shifts are reshaping competitive positioning and creating new opportunities for integrated solutions that pair devices with data-driven clinical support.
Assessing the cumulative impact of United States tariffs introduced in 2025 on supply chains, procurement strategies, pricing dynamics and clinical access to devices
The 2025 tariff measures implemented by the United States have introduced a structural friction that affects device manufacturers, distributors, and health systems in different ways. For companies that rely on cross-border manufacturing and component sourcing, tariffs have increased the complexity of sourcing strategies and elevated the importance of supply chain transparency. Consequently, manufacturers have accelerated efforts to diversify supplier bases, nearshore production, and revisit bill-of-materials choices to mitigate exposure to trade-related cost volatility.
Procurement teams within hospitals and ambulatory surgical centers are responding by shifting from unit-price negotiations toward total cost of ownership conversations that encompass inventory carrying costs, logistics lead times, and contingency stock policies. These negotiations often favor suppliers who can demonstrate resilient supply chains and flexible fulfillment options. In parallel, distributors are reevaluating their inventory strategies, adjusting buffer stocks, and seeking contractual frameworks that share tariff-related risks with manufacturers or purchasers.
Clinicians and health system leaders are also attuning to potential access implications. In settings where device selection is tightly coupled to reimbursement or bundled care pathways, tariff-driven cost dynamics could influence formularies or preferred vendor lists, indirectly shaping clinical practice. To respond, stakeholders across the value chain are investing in scenario planning and contracting innovations that preserve clinical choice while addressing affordability. Regulatory compliance, customs classification, and tariff engineering have thus become integral components of commercialization strategy rather than peripheral administrative tasks.
Key segmentation insights across product technologies, procedural contexts, end users and sales channels informing differentiated go-to-market and R&D strategies
A granular segmentation lens reveals how product characteristics, procedural context, end-user environment, and distribution channels interact to shape clinical adoption and commercial performance. Within product modalities, coils remain a foundational technology and are differentiated across bare platinum, bioactive, and hydrogel-coated variants; the hydrogel-coated category further divides into multi-layer and single-layer architectures. Each coil subtype presents distinct handling characteristics, occlusion profiles, and implications for retreatment risk, which influence physician preference and inventory management. Flow diverters have emerged as a separate class for select wide-neck and fusiform aneurysms, introducing different sizing considerations and follow-up paradigms. Liquid embolics, including formulations such as NBCA and Onyx, offer a complementary approach for certain anatomies and present unique delivery system requirements and training needs.
Procedure type exerts a strong influence on device selection and operational planning. Elective cases allow for pre-procedural planning, case-specific device preparation, and scheduled allocation of specialized personnel, while emergency procedures demand rapid access to a range of compatible devices and streamlined decision pathways to enable timely intervention. The end-user environment-whether an ambulatory surgical center, a hospital, or a specialty clinic-further conditions logistical capabilities, case mix, and the available peri-procedural support infrastructure, which in turn shapes preferred device profiles and service-level expectations.
Finally, sales channels mediate how devices reach end users and how commercial relationships are managed. Direct sales models facilitate deeper clinical engagement and tailored training programs that can accelerate adoption of novel technologies, whereas distributor-based models offer scale and regional reach but may require different incentive and support structures. Understanding the interplay between product complexity, procedure urgency, care setting, and distribution pathways is essential for designing differentiated go-to-market strategies and for prioritizing evidence generation that resonates with clinical and procurement stakeholders.
Regional dynamics shaping adoption and access across Americas, Europe, Middle East & Africa, and Asia-Pacific with implications for clinical practice and market entry
Geographic dynamics exert a profound influence on clinical practice patterns, regulatory expectations, and commercial strategies for aneurysm coiling and embolization devices. In the Americas, advanced tertiary centers drive early adoption of novel technologies and high-volume procedural experience, which supports robust registries and longitudinal outcome data collection. Reimbursement mechanisms and centralized purchasing in many jurisdictions shape pricing dynamics and favor devices that demonstrably reduce retreatment or intensive care utilization. As a result, manufacturers often prioritize evidence packages and post-market studies that align with payer priorities in this region.
In Europe, Middle East & Africa, heterogeneity in regulatory frameworks and healthcare financing creates a varied landscape where pathway-specific adoption is common. Some markets emphasize rapid regulatory review and centralized procurement, while others maintain localized decision-making that rewards targeted clinical engagement and adaptable distribution models. Clinical guidelines and national HTA processes in parts of Europe also shape the timing and extent of uptake, making alignment with evidence requirements critical for market entry and expansion.
Asia-Pacific presents a mixed picture of high-growth potential and diverse market structures. Several countries in this region are investing heavily in neurointerventional capabilities, expanding procedural volumes in tertiary hospitals and specialty centers. Local manufacturing and cost-sensitivity influence procurement choices, and partnerships with regional distributors or local manufacturers can accelerate market penetration. Cultural and clinical practice variations also affect device selection and follow-up strategies, requiring tailored training programs and region-specific clinical data to support adoption. Across regions, consistent themes include the primacy of clinical evidence, the importance of supply chain resilience, and the need to align commercial models with local healthcare delivery realities.
Competitive landscape and company-level insights highlighting strategic partnerships, innovation pipelines, regulatory positioning and commercialization tactics
The competitive environment in aneurysm coiling and embolization is characterized by a mix of established medical technology firms, specialist neurovascular device manufacturers, and agile start-ups focused on niche innovations. Established firms leverage broad commercial footprints, integrated sales forces, and comprehensive service offerings to maintain access to major health systems and to support large-scale evidence generation. Specialist manufacturers often compete on the basis of distinct technological advantages, procedural ease-of-use, or differentiated clinical data that address clear unmet needs in specific aneurysm subtypes.
Start-ups and smaller innovators play an essential role in introducing disruptive concepts, whether through novel coil materials, next-generation flow diverter designs, or improved liquid embolic chemistries and delivery systems. These entrants commonly pursue targeted clinical collaborations and registry-driven evidence paths to validate performance before scaling. Their agility enables rapid iteration, but they face challenges in scaling manufacturing, navigating regulatory pathways, and building reimbursement support.
Strategic partnerships, licensing agreements, and acquisitions are recurring themes as companies seek to combine complementary capabilities-such as a novel device platform paired with an incumbent’s distribution infrastructure. Contract manufacturing organizations and specialized supply chain partners also play a pivotal role by enabling cost-effective scale-up. Overall, success in this space requires alignment among product innovation, clinical evidence generation, regulatory strategy, and a commercial model that supports education and long-term follow-up.
Actionable recommendations for industry leaders to optimize product portfolios, strengthen supply chains, and accelerate clinical adoption while managing regulatory risk
Industry leaders should pursue a set of integrated actions that balance innovation with operational resilience and stakeholder alignment. First, prioritize development and validation of device features that address clinically meaningful outcomes such as reduced retreatment rates, improved occlusion durability, and streamlined procedural workflows; coupling these technical advances with prospective registry data will strengthen clinical adoption. Second, invest in targeted education and competency programs that shorten the learning curve for complex devices and embed product champions within high-volume centers, leveraging simulation and proctoring to reinforce best practices.
Third, strengthen supply chain robustness by diversifying suppliers, considering nearshoring where appropriate, and negotiating contracting terms that share tariff and logistic risks; this reduces vulnerability to trade disruptions and supports reliable availability for emergency cases. Fourth, adapt commercial models to the care setting: direct engagement with high-volume hospitals and specialty clinics can justify premium positioning through service bundles, while distributor partnerships remain effective for broader geographic reach and cost-sensitive segments. Fifth, align reimbursement and health economics activities early by generating the evidence that payers and procurement teams require to evaluate total cost of care, length of stay, and downstream utilization.
Finally, consider strategic collaborations that accelerate clinical evidence generation and market access, including partnerships with academic centers for investigator-led trials and alliances with regional distributors to tailor logistical solutions. These combined actions will help companies translate product innovation into sustained clinical adoption and commercial success while managing regulatory and policy headwinds.
Robust research methodology combining clinical evidence review, stakeholder interviews, regulatory analysis and supply chain mapping to validate insights and recommendations
The research underpinning this executive summary synthesizes multiple evidentiary streams to ensure robust, validated insights. The methodology included a structured review of peer-reviewed clinical literature, major clinical guidelines, and registry publications to capture the latest evidence regarding safety, efficacy, and long-term outcomes across device classes. Supplementing this, the team conducted in-depth interviews with a cross-section of stakeholders, including interventional neuroradiologists, neurosurgeons, hospital procurement leads, and distributor partners, to surface real-world operational considerations, training needs, and procurement dynamics.
Regulatory analysis examined public dossiers, approval timelines, and post-market surveillance requirements across key jurisdictions to identify differences in evidence expectations and compliance obligations. Supply chain mapping involved tracing component sourcing and manufacturing footprints, reviewing logistics pathways, and assessing the implications of trade and tariff changes on procurement practices. Commercial channel analysis compared direct sales and distributor models, focusing on training, service delivery, and contractual frameworks that influence adoption.
Finally, all findings were triangulated through a validation workshop with domain experts to test hypotheses and refine recommendations. This multi-method approach-integrating quantitative literature synthesis, qualitative stakeholder insight, regulatory review, and supply chain analysis-ensures that conclusions reflect both the published evidence base and the practical realities of delivering aneurysm care.
Conclusion synthesizing strategic implications for clinicians, manufacturers, payers and distributors to navigate technological change and policy headwinds effectively
Modern neurointerventional practice sits at the intersection of substantive device innovation, evolving clinical evidence, and complex commercial dynamics. As therapy options diversify across coils, flow diverters, and liquid embolics, decision-makers must integrate device-specific performance characteristics with procedural urgency, institutional capabilities, and payer expectations. The cumulative effects of trade policy adjustments, changing procurement strategies, and regional regulatory diversity further complicate commercialization and access planning.
Going forward, the organizations that will succeed are those that align product design with rigorous outcomes data, invest in scalable training and support, and construct resilient supply chains that can withstand geopolitical and logistical shocks. Strategic collaboration-whether through partnerships, targeted acquisitions, or academic alliances-will accelerate evidence generation and broaden market access. At the same time, adaptive commercial models that tailor engagement to care settings and leverage both direct and distributor channels will be essential for meeting diverse customer needs.
In sum, the aneurysm coiling and embolization device ecosystem is poised for continued evolution. Stakeholders who synthesize clinical insight with pragmatic operational strategies and proactive regulatory planning will be best positioned to deliver improved patient outcomes while achieving sustainable commercial performance.
Additional Product Information:
- Purchase of this report includes 1 year online access with quarterly updates.
- This report can be updated on request. Please contact our Customer Experience team using the Ask a Question widget on our website.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
16. China Aneurysm Coiling & Embolization Devices Market
Companies Mentioned
The key companies profiled in this Aneurysm Coiling & Embolization Devices market report include:- Abbott Laboratories
- Acandis GmbH
- B. Braun Melsungen AG
- BALT Extrusion S.A.
- Boston Scientific Corporation
- Cook Medical LLC
- Johnson & Johnson
- KANEKA CORPORATION
- Medtronic plc
- MicroPort Scientific Corporation
- MicroVention, Inc.
- Penumbra, Inc.
- Shape Memory Medical Inc.
- Stryker Corporation
- Terumo Corporation
- W. L. Gore & Associates, Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 180 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
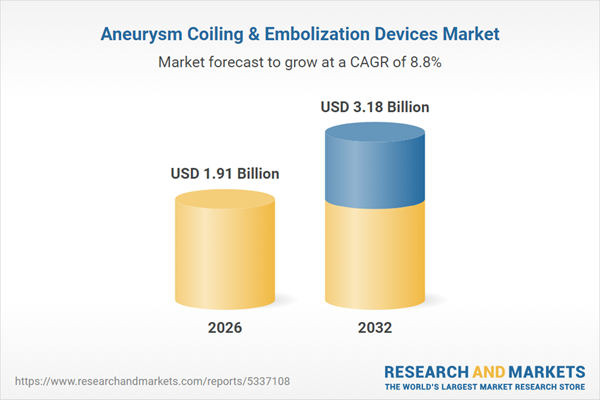
| Estimated Market Value ( USD | $ 1.91 Billion |
| Forecasted Market Value ( USD | $ 3.18 Billion |
| Compound Annual Growth Rate | 8.7% |
| Regions Covered | Global |
| No. of Companies Mentioned | 17 |