The Alzheimer’s disease Therapeutics Pipeline is driven by an increasing gap of unmet need of effective therapeutics that can cure Alzheimer’s disease. Additionally, life expectancy has increased due to various healthcare reforms in major economies such as the U.S., Japan, and China. This has resulted in a large geriatric population which is the age group most affected by the disease.
Alzheimer’s prevalence is rising rapidly and despite decades of research, the disease remains incurable. The majority of therapeutics target symptom reduction and slowing the progression of the disease. The Food and Drug Administration (FDA) approved aducanumab (Aduhelm) for the treatment of certain cases of Alzheimer's disease in June 2021 and this is the first drug approved for Alzheimer's disease in decades.
Eisai, Biogen, Hoffmann-La Roche, AZTherapies, Cerecin, Neurotrope, AC Immune, Cassava Sciences, AB Science, Anavex Life Sciences, Athira Pharma, Denali Therapeutics Inc., and other notable companies are developing therapeutic candidates to improve the Alzheimer's Diagnosis and treatment scenario.
Scope of the Report
- The report analyses the Alzheimer’s Disease Therapeutics in Pipeline by Molecule Type (Small Molecules, Monoclonal Antibody, Vaccine, DNA/RNA Based, Natural products, Gene Therapy, Cell Therapy, Others).
- The report analyses the Alzheimer’s disease Therapeutics by Route of Administration (Oral, Intravenous, Intramuscular, Subcutaneous, Intrathecal, Intranasal, Other).
- The report analyses the Alzheimer’s disease Therapeutics by Pipeline Phase (Phase I, Phase I/II, Phase II, Phase II/III, Phase III, Phase IV, Preclinical).
- The report analyses the Pain Management Drugs Market by Availability (Over the Counter (OTC), Prescription).
- The Alzheimer’s Disease Therapeutics- Pipeline Analysis has been analysed By Region (Americas, Europe, Asia Pacific, and MEA).
- The Global Pain Management Drugs Market has been analysed By Region (Americas, Europe, Asia Pacific, and MEA).
- The key insights of the report have been presented through the leading company shares.
- Also, the major, trends, drivers and challenges as well as Unmet Needs of the industry has been analysed in the report.
- The companies analysed in the report include Eli-Lily & Co , Elsal Co., Ltd., BioVie, Johnson and Johnson, Otsuka Pharmaceutical Co., Ltd. H. Lundbeck A/S, Novartis, Cognition Therapeutics, Merck Sharp & Dohme LLC, Biogen.
Key Target Audience
- Alzheimer’s Disease Therapeutics Companies
- End Users (Hospitals and clinics)
- Research and Development (R&D) Organizations
- Government Bodies & Regulating Authorities
- Investment Banks and Equity Firms
Table of Contents
1. Introduction
1.1 Alzheimer's Disease Therapeutics Overview
1.2 Scope of Research
2. Executive Summary
2.1 Market Dashboard
2.2 Regional Insights
2.3 Market Ecosystem Factors
3. Research Methodology
3.1 Data Collection Process
3.2 Market Size Calculation-Top-to-Bottom
4. Macro Economic Indicator Outlook
4.1 Global, Region wise GDP Growth
4.2 Global Medical Spending
4.3 Current Healthcare Expenditure
4.4 Pharmaceutical Spending/capita
5. Competitive Positioning
5.1 Companies’ Product Positioning
5.2 Competitive positioning
5.2.1 Eli-Lily & Co
5.2.2 Elsal Co., Ltd.
5.2.3 BioVie
5.2.4 Johnson & Johnson
5.2.5 Otsuka Pharmaceutical Co., Ltd.
5.2.6 H. Lundbeck A/S
5.2.7 Novartis
5.2.8 Cognition Therapeutics
5.2.9 Merck Sharp & Dohme LLC
5.2.10 Biogen
6. Alzheimer’s disease background
6.1 AD Fact Sheet
6.2 Epidemiology
6.3 Causes and Risk Factors
6.4 Diagnosis and Assessment
6.5 Unmet Needs
7. Therapeutics in Pipeline
7.1 Pipeline Scenario
7.2 Alzheimer’s Therapeutics Comparative Review
8. Pipeline Analysis
9. Phase I therapeutics Overview
9.1 LY3372993
9.1.1 Drug Description
9.1.2 Outcomes
9.2 Lu AF87908
9.2.2 Drug Description
9.2.3 Outcomes
9.3 anle138b
9.3.1 Drug Description
9.3.2 Outcomes
9.3.3 Collaborators
9.4 ASN51
9.4.1 Drug Description
9.4.2 Outcomes
9.4.3 Collaborators
9.5 CMS121
9.5.1 Drug Description
9.5.2 Outcomes
9.5.3 Collaborators
9.6 IBC-Ab002
9.6.1 Drug Description
9.6.2 Outcomes
9.6.3 Collaborators
9.7 LX1001
9.7.1 Drug Description
9.7.2 Outcomes
9.8 MK-2214
9.8.1 Drug Description
9.8.2 Outcomes
9.9 MK-8189
9.9.1 Drug Description
9.9.2 Outcomes
9.10 ACU193
9.10.1 Drug Description
9.10.2 Outcomes
9.10.3 Collaborators
9.11 NIO752
9.11.1 Drug Description
9.11.2 Outcomes
9.12 ALN-APP
9.12.1 Drug Description
9.12.2 Outcomes
9.13 TB006
9.13.1 Drug Description
9.13.2 Outcomes
9.14 SNK01
9.14.1 Drug Description
9.14.2 Outcomes
9.15 Pepinemab
9.15.1 Drug Description
9.15.2 Outcomes
9.15.3 Collaborators
9.16 GB-5001
9.16.1 Drug Description
9.16.2 Outcomes
9.17 AC-1202
9.17.1 Drug Description
9.17.2 Outcomes
9.18 SHR-1707
9.18.1 Drug Description
9.18.2 Outcomes
9.19 BEY2153
9.19.1 Drug Description
9.19.2 Outcomes
9.20 JNJ-40346527
9.20.1 Drug Description
9.20.2 Outcomes
9.20.3 Collaborators
9.21 APNmAb005
9.21.1 Drug Description
9.21.22 Outcomes
9.22 NPT 2042
9.22.1 Drug Description
9.22.2 Outcomes
9.23 ALZ-101
9.23.1 Drug Description
9.23.2 Outcomes
10. Phase I/II therapeutics Overview
10.1 Posiphen
10.1.1 Drug Description
10.1.2 Outcomes
10.1.3 Collaborators
10.2 PrimePro™/ PrimeMSK™
10.2.1 Drug Description
10.2.2 Outcomes
10.2.3 Collaborators
10.3 TB006
10.3.1 Drug Description
10.3.2 Outcomes
10.4 RO7126209
10.4.1 Drug Description
10.4.2 Outcomes
10.5 DNL593
10.5.1 Drug Description
10.5.2 Outcomes
10.5.3 Collaborators
10.6 BIIB080
10.6.1 Drug Description
10.6.1 Outcomes
10.7 E2814
10.7.1 Drug Description
10.7.2 Outcomes
10.8 Tdap
10.8.1 Drug Description
10.8.2 Outcomes
10.8.3 Collaborators
10.9 ACI-35.030/ JACI-35.054
10.9.1 Drug Description
10.9.2 Outcomes
10.9.3 Collaborators
10.10 ACI-24.060
10.10.1 Drug Description
10.10.2 Outcomes
10.10.3 Collaborators
10.11 IVL3003
10.10.1 Drug Description
10.10.2 Outcomes
11. Phase II therapeutics Overview
11.1 Lecanemab
11.1.1 Drug Description
11.1.2 Outcomes
11.1.3 Collaborators
11.2 Lomecel-B
11.2.1 Drug Description
11.2.2 Outcomes
11.2.3 Collaborators
11.3 AL002
11.3.1 Drug Description
11.3.2 Outcomes
11.3.3 Collaborators
11.4 AL001
11.4.1 Drug Description
11.4.2 Outcomes
11.5 CT1812 (Elaya)
11.5.1 Drug Description
11.5.2 Outcomes
11.6 APH-1105
11.6.1 Drug Description
11.6.2 Outcomes
11.7 ATH-1017
11.7.1 Drug Description
11.7.2 Outcomes
11.8 T-817MA
11.8.1 Drug Description
11.8.2 Outcomes
11.9 Montelukast buccal film
11.9.1 Drug Description
11.9.2 Outcomes
11.10 ABBV-916
11.10.1 Drug Description
11.10.2 Outcomes
11.11 Bepranemab
11.11.1 Drug Description
11.11.2 Outcomes
11.12 TB006
11.12.1 Drug Description
11.12.2 Outcomes
11.13 LY3372689
11.13.1 Drug Description
11.13.2 Outcomes
11.14 MW150
11.14.1 Drug Description
11.14.2 Outcomes
11.14.3 Collaborators
11.15 EX039
11.15.1 Drug Description
11.15.2 Outcomes
11.15.3 Collaborators
11.16 JNJ-42847922
11.16.1 Drug Description
11.16.2 Outcomes
11.17 REM0046127
11.17.1 Drug Description
11.17.2 Outcomes
11.18 Bryostatin 1
11.18.1 Drug Description
11.18.2 Outcomes
11.18.3 Collaborators
11.19 NanoLithium® NP03
11.19.1 Drug Description
11.19.2 Outcomes
11.20 AstroStem
11.20.1 Drug Description
11.20.2 Outcomes
11.21 Gantenerumab
11.21.1 Drug Description
11.21.2 Outcomes
11.22 Semorinemab
11.22.1 Drug Description
11.22.1 Outcomes
11.23 Obicetrapib
11.23.1 Drug Description
11.23.2 Outcomes
11.24 ALZ-801
11.24.1 Drug Description
11.24.2 Outcomes
11.25 CY6463
11.25.1 Drug Description
11.25.2 Outcomes
11.26 Crenezumab
11.26.1 Drug Description
11.26.2 Outcomes
11.26.3 Collaborators
11.27 T3D-959
11.27.1 Drug Description
11.27.2 Outcomes
11.28 PMZ-1620
11.28.1 Drug Description
11.28.2 Outcomes
11.29 GV1001
11.29.1 Drug Description
11.29.2 Outcomes
11.30 ACZ885
11.30.1 Drug Description
11.30.2 Outcomes
11.31 PQ912
11.31.1 Drug Description
11.31.2 Outcomes
11.31.3 Collaborators
11.32 APH-1105
11.32.1 Drug Description
11.32.2 Outcomes
11.33 SLS-005
11.33.1 Drug Description
11.33.2 Outcomes
11.33.3 Collaborators
11.34 Flos gossypii flavonoids
11.34.1 Drug Description
11.34.2 Outcomes
11.34.3 Collaborators
11.35 Human Mesenchymal Stem cells (MSCs),
11.35.1 Drug Description
11.35.2 Outcomes
11.36 JNJ-63733657
11.36.1 Drug Description
11.36.2 Outcomes
11.37 IGC-AD1
11.37.1 Drug Description
11.37.2 Outcomes
11.38 TW001
11.38.1 Drug Description
11.38.2 Outcomes
11.39 ABvac40
11.39.1 Drug Description
11.39.2 Outcomes
12. Phase II/III therapeutics Overview
12.1 Tricaprilin
12.1.1 Drug Description
12.1.2 Outcomes
12.2 ANAVEX2-73
12.2.1 Drug Description
12.2.3 Outcomes
12.3 Piromelatine
12.3.1 Drug Description
12.3.2 Outcomes
12.3.3 Collaborators
12.4 AGB101
12.4.1 Drug Description
12.4.2 Outcomes
12.4.3 Collaborators
13. Phase III therapeutics Overview
13.1 Simufilam
13.1.1 Drug Description
13.1.2 Outcomes
13.1.3 Collaborators
13.2 ATH-1017
13.2.1 Drug Description
13.2.2 Outcomes
13.3 Lecanemab
13.3.1 Drug Description
13.3.2 Outcomes
13.3.3 Collaborators
13.4 Nilotinib BE
13.4.1 Drug Description
13.4.2 Outcomes
13.4.3 Collaborators
13.5 Brexpiprazole
13.5.1 Drug Description
13.5.2 Outcomes
13.5.3 Collaborators
13.6 Masitinib
13.6.1 Drug Description
13.6.2 Outcomes
13.7 Remternetug
13.7.1 Drug Description
13.7.2 Outcomes
13.8 Donanemab
13.8.1 Drug Description
13.8.2 Outcomes
13.9 NE3107
13.9.1 Drug Description
13.9.2 Outcomes
13.10 Gantenerumab
13.10.1 Drug Description
13.10.2 Outcomes
13.11 GV-971
13.11.1 Drug Description
13.11.2 Outcomes
13.12 Aducanumab
13.12.1 Drug Description
13.12.2 Outcomes
13.13 TRx0237
13.13.1 Drug Description
13.13.2 Outcomes
13.14 Semagludtide
13.14.1 Drug Description
13.14.2 Outcomes
13.15 BPDO-1603
13.15.1 Drug Description
13.15.2 Outcomes
13.16 AR1001
13.16.1 Drug Description
13.16.2 Outcomes
13.17 KarXT
13.17.1 Drug Description
13.17.2 Outcomes
13.18 AXS-05
13.18.1 Drug Description
13.18.2 Outcomes
14. Phase IV therapeutics Overview
14.1 Choline Alfoscerate
14.1.1 Drug Description
14.1.2 Outcomes
14.2 Ebicomb
14.2.1 Drug Description
14.2.2 Outcomes
14.3 Rivastigmine
14.3.1 Drug Description
14.3.2 Outcomes
14.4 GV-971
14.4.1 Drug Description
14.4.2 Outcomes
15. Preclinical Phase therapeutics Overview
15.1 PMN310
15.1.1 Drug Description
15.1.2 Outcomes
15.1.3 Collaborators
15.2 PRX123
15.2.1 Drug Description
15.2.2 Outcomes
16. Pipeline analysis, By molecule type
16.1 Alzheimer’s Therapeutics Comparative Review, by Molecule type
17. Pipeline analysis, By Route of Administration
17.1 Alzheimer’s Therapeutics Comparative Review, By Route of Administration
18. About the Publisher
List of Figures
1: Alzheimer's Disease Therapeutics for next 7 years (in USD Millions)
2: Alzheimer's Disease Therapeutics Size, By Sales Channel, 2018 & 2028 (USD Million) 3: Alzheimer’s Hospitalization Statistics, 2022 (per 1,000 Medicare Beneficiaries)
4: Eli Lilly and Company Revenues, 2019-2021 (USD Million)
5: Eli Lilly and Company Revenue, By Business Segments, 2021 (%)
6: Eli Lilly and Company Revenue, By Geographical Segments, 2021 (%)
7: Eisai Co., Revenues, 2019-2021 (USD Million)
8: Eisai Co., Revenue, By Business Segments, 2021 (%)
9: Eisai Co., Revenue, By Geographical Segments, 2021 (%)
10: Johnson & Johnson Revenues, 2019-2021 (USD Million)
11: J&J Revenue, By Business Segments, 2021 (%)
12: J&J Revenue, By Geographical Segments, 2021 (%)
13: Otsuka Pharmaceuticals Revenues, 2019-2021 (USD Million)
14: Otsuka Revenue, By Business Segments, 2021 (%)
15: Otsuka Revenue, By Business Segments, 2020 (%)
16: H. Lundbeck Revenues, 2019-2021 (USD Million)
17: H. Lundbeck Revenue, By Business Segment by Product, 2021 (%)
18: H. Lundbeck Revenue, By Geographical Segments, 2021 (%)
19: Novartis Revenues, 2019-2021 (USD million)
20: Novartis Revenue, By Business Segments, 2021 (%)
21: Novartis Revenue, By Geographic Segments, 2021 (%)
22: Merck & Co., Inc. Revenues, 2019-2021 (USD Million)
23: Merck & Co., Inc. Revenue, By Business Segments, 2021 (%)
24: Merck & Co., Inc. Revenue, By Geographic segment, 2021 (%)
25: Biogen Revenues, 2019-2021 (USD Million)
26: Biogen Revenue, By Business Segments - By Products, 2021 (%)
27: Biogen Revenue, By Geographic Segments, 2021 (%)
28: Global Prevalence of Alzheimer's disease and other dementias, 2017-2019 (Millions)
29: Global Incidence of Alzheimer's disease and other dementias, 2017-2019 (Millions)
30: Global Prevalence of Alzheimer's disease and other dementias, In Females, 2017-2019 (Millions)
31: Global Prevalence of Alzheimer's disease and other dementias, In Males, 2017-2019 (Millions)
32: Global Diabetes data report 2000 - 2045(E), (in thousands)
33: Estimated global prevalence and numbers of people with obesity and severe obesity in 2020, 2025 (E) and 2030 (E)
34: Estimated prevalence of obesity amongst women in, 2030 (E)
35: Estimated prevalence of obesity amongst men in, 2030 (E)
36: Therapeutics in Phase I, by Molecule type
37: Therapeutics in Phase I/II by Molecule type
38: Therapeutics in Phase II by Molecule type
39: Therapeutics in Phase II/III by Molecule type
40: Therapeutics in Phase III by Molecule type
41: Therapeutics in Phase IV by Molecule type
42: Therapeutics in Preclinical Phase, by Molecule type
List of Tables
1: Key Company Financials, 2019-2021
2: Key Company Financials, 2019-2021
3: Key Company Financials, 2019-2021
4: Key Company Financials, 2019-2021
5: Key Company Financials, 2019-2021
6: Key Company Financials, 2019-2021
7: Key Company Financials, 2019-2021
8: Key Company Financials, 2019-2021
9: Total Drugs in Alzheimer’s Disease Pipeline
10: Total Alzheimer’s Disease Drugs in Phase I of Pipeline
11: Total Alzheimer’s Disease Drugs in Phase I/II of Pipeline
12: Total Alzheimer’s Disease Drugs in Phase II of Pipeline
13: Total Alzheimer’s Disease Drugs in Phase II/III of Pipeline
14: Total Alzheimer’s Disease Drugs in Phase III of Pipeline
15: Total Alzheimer’s Disease Drugs in Phase IV of Pipeline
16: Total Alzheimer’s Disease Drugs in Preclinical Phase of Pipeline
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 200 |
| Published | October 2022 |
| Forecast Period | 2021 - 2028 |
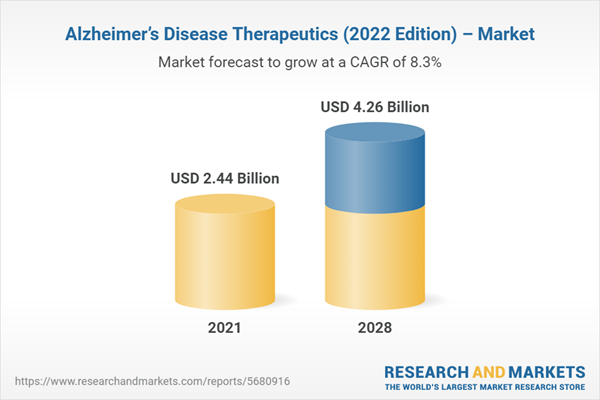
| Estimated Market Value ( USD | $ 2.44 Billion |
| Forecasted Market Value ( USD | $ 4.26 Billion |
| Compound Annual Growth Rate | 8.3% |
| Regions Covered | Global |