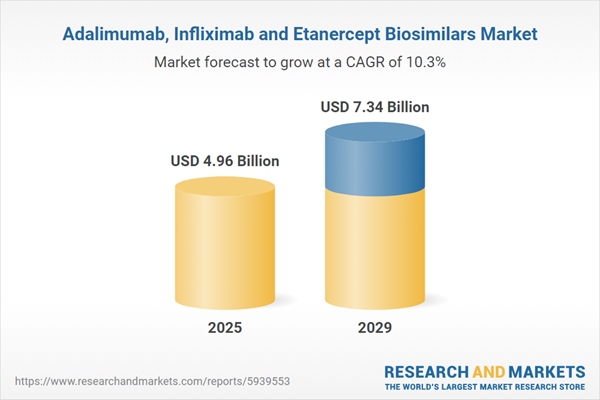
The adalimumab, infliximab and etanercept biosimilars market size is expected to see rapid growth in the next few years. It will grow to $7.34 billion in 2029 at a compound annual growth rate (CAGR) of 10.3%. The growth in the forecast period can be attributed to a rise in healthcare expenditure, an aging population and an increase in healthcare access will drive market growth. Major trends in the forecast period include focus on launching new products, focus on mergers and acquisitions, focus on establishing strategic partnerships, and focus on increasing investments.
The forecast of 10.3% growth over the next five years reflects a modest reduction of 0.3% from the previous estimate for this market. This reduction is primarily due to the impact of tariffs between the US and other countries. Trade tensions could hinder U.S. rheumatology and gastroenterology practices by inflating prices of TNF-alpha inhibitor biosimilars (adalimumab, infliximab, etanercept) manufactured in South Korea and India, resulting in delayed autoimmune disease treatment and higher biologic therapy costs. The effect will also be felt more widely due to reciprocal tariffs and the negative effect on the global economy and trade due to increased trade tensions and restrictions.
The entrance of biosimilars into the market for adalimumab, infliximab, and etanercept is facilitated by the patent expiration of branded drugs. Notably, the FDA approved Hospira's Inflectra post the patent expiry of the branded drug Remicade, enabling treatment for various autoimmune diseases such as rheumatoid arthritis, adult ulcerative colitis, and plaque psoriasis in the United States. Similarly, in the EU, Amgen's Enbrel patent expiration led to the approval of Benepali, a biosimilar of Enbrel, by the European Commission. Following the expiry of Humira's patent, multiple biosimilars have become available in the market, including the recent approval (July 2020) of Hulio, the sixth biosimilar of Humira by the FDA. Consequently, the patent expiry of branded biologic drugs such as Humira, Enbrel, and Remicade is expected to propel demand in the adalimumab, infliximab, and etanercept biosimilars market.
The escalating prevalence of autoimmune diseases is anticipated to drive growth in the market for adalimumab, infliximab, and etanercept biosimilars. Autoimmune diseases arise from immune system dysfunction, leading immune cells to attack the body's healthy cells. Adalimumab, infliximab, and etanercept, as biological drugs with tumor necrosis factor (TNF) alpha inhibitors, are employed in treating various autoimmune diseases. According to Johns Hopkins University in 2023, approximately 10 million individuals in the US, constituting 3% of the population, are affected by diverse autoimmune illnesses. Consequently, the surge in autoimmune diseases' prevalence significantly contributes to driving the growth of the adalimumab, infliximab, and etanercept biosimilars market.
The brand-name versions of biosimilar drugs are expensive, and many are now being replaced by biosimilar versions following the expiration of their patents. The costs of branded biological drugs have nearly doubled in recent years. For example, in September 2022, the biologic medication Humira, used to treat autoimmune conditions such as rheumatoid arthritis and Crohn's disease, was priced at over $6,000 for a carton containing two pens without insurance. The transition to lower-cost biosimilar drugs is primarily due to the absence of clinically significant differences in safety and efficacy between the biosimilars and the original biologics. After the patent on the original adalimumab product, Humira, expired, the Danish healthcare system nearly completely transitioned to adalimumab biosimilars, resulting in an 82% reduction in medication costs.
Major companies in the adalimumab, infliximab, and etanercept biosimilars market are prioritizing the development of innovative products, such as citrate-free adalimumab, to offer enhanced services to consumers. Citrate-free adalimumab represents a formulation of the biological medication adalimumab devoid of citrate. For instance, in December 2022, Fresenius Kabi, a German-based manufacturer specializing in medicines and technologies for infusion, transfusion, and clinical nutrition, announced FDA approval for the biosimilar Idacio (adalimumab). This biosimilar is sanctioned for use in managing chronic autoimmune disorders across all permissible uses of the reference product. It has been meticulously crafted employing advanced analytical methodologies, intended for the treatment of various chronic conditions.
In February 2022, the Indian biopharmaceutical company Biocon Limited successfully acquired the biosimilar assets of Viatris, Inc., a U.S.-based biopharmaceutical and healthcare company, for a total of $3.34 billion. This strategic acquisition positions Biocon as a unique and vertically integrated global leader in biosimilars, providing an impetus for the expedited commercialization of its existing and future biosimilar portfolio on a global scale. The move is set to significantly enhance Biocon's product reach across international markets. Viatris, Inc. is recognized for its expertise in the development and specialization of biological and therapeutic agents.
Major companies operating in the adalimumab, infliximab and etanercept biosimilars market include Biogen, Novartis (Sandoz), Pfizer, Amgen, Celltrion, Samsung Bioepis (Samsung Biologics), Hetero Drugs Limited, Fresenius Kabi AG, Boehringer Ingelheim, Biocon, Zydus Lifesciences Limited, Reliance Life Sciences, Torrent Pharmaceuticals Ltd, Sun Pharmaceutical Industries Ltd, Cipla Limited, Zhejiang Hisun Pharmaceutical Co. Ltd, Bio-Thera Solutions, Ltd, Innovent Biologics (Suzhou) Co. Ltd, Janssen Biologics BV, Shanghai CP Guojian Pharmaceutical Co. Ltd, Sansheng Guojian, Shanghai Junshi Biosciences Co. Ltd, Mabwell Bioscience Co. Ltd, LG Chem, Nippon Kayaku, Mochida Pharmaceutical Co. Ltd, mAbxience, Allergan, Microgen, Geropharm, Valenta, NovaMedica Veropharm, Biocad, Roche, Bristol-Myers Squibb, Celon Pharma, SynBio, Ache, Eurofarma, Teva Pharmaceuticals, Oramed Pharmaceuticals, Entera Bio, Julphar, Hikma Pharmaceuticals, Mylan, BIOPHARMA-MEA, NeoTX, AID Genomics Limited, Altis Biologics, Next Biosciences, Viome, Inqaba Biotechnical Industries (Pty) Ltd.
North America was the largest region in the adalimumab, infliximab, and etanercept biosimilars market in 2024. The regions covered in the adalimumab, infliximab, and etanercept biosimilars market report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East, Africa. The countries covered in the adalimumab, infliximab and etanercept biosimilars market report are Australia, Brazil, China, France, Germany, India, Indonesia, Japan, Russia, South Korea, UK, USA, Italy, Canada, Spain.
Note that the outlook for this market is being affected by rapid changes in trade relations and tariffs globally. The report will be updated prior to delivery to reflect the latest status, including revised forecasts and quantified impact analysis. The report’s Recommendations and Conclusions sections will be updated to give strategies for entities dealing with the fast-moving international environment.
The sudden escalation of U.S. tariffs and the resulting trade tensions in spring 2025 are having a significant impact on the pharmaceutical sector. Companies are grappling with higher costs on imported active pharmaceutical ingredients (APIs), glass vials, and laboratory equipment - many of which have limited alternative sources. Generic drug manufacturers, already operating with minimal profit margins, are particularly affected, with some scaling back production of low-margin medications. Biotech firms are also experiencing delays in clinical trials due to shortages of specialized reagents linked to tariffs. In response, the industry is shifting API production to regions like India and Europe, building up inventory reserves, and advocating for tariff exemptions on essential medicines.
The adalimumab, infliximab, and etanercept biosimilars market research report is one of a series of new reports that provides adalimumab, infliximab, and etanercept biosimilars market statistics, including adalimumab, infliximab, and etanercept biosimilars industry global market size, regional shares, competitors with adalimumab, infliximab, and etanercept biosimilars market. This adalimumab, infliximab, and etanercept biosimilars market research report delivers a complete perspective of everything you need, with an in-depth analysis of the current and future scenarios of the industry.
Adalimumab, infliximab, and etanercept biosimilars represent an innovative class of medications designed to inhibit the effects of the inflammatory mediator tumor necrosis factor-alpha (TNF-alpha). Infliximab biosimilars, in particular, are chimeric monoclonal antibodies targeting TNF-alpha and are utilized in the treatment of immune system disorders.
These medications include various products such as adalimumab, infliximab, cipleumab, and etanercept. Adalimumab, classified as a biological drug, operates on the immune system to mitigate inflammation. Its diverse applications encompass treating conditions such as Crohn’s disease, psoriatic arthritis, rheumatoid arthritis, ulcerative colitis, ankylosing spondylitis, plaque psoriasis, and others. These products are made available through different distribution channels, including hospital pharmacies, retail pharmacies, and online pharmacies.
The adalimumab, infliximab, and etanercept biosimilars market consists of sales of Amgevita, Hyrimoz, and Idacio. Values in this market are factory gate values, that is, the value of goods sold by the manufacturers or creators of the goods, whether to other entities (including downstream manufacturers, wholesalers, distributors, and retailers) or directly to end customers. The value of goods in this market includes related services sold by the creators of the goods.
The market value is defined as the revenues that enterprises gain from the sale of goods and/or services within the specified market and geography through sales, grants, or donations in terms of the currency (in USD, unless otherwise specified).
The revenues for a specified geography are consumption values that are revenues generated by organizations in the specified geography within the market, irrespective of where they are produced. It does not include revenues from resales along the supply chain, either further along the supply chain or as part of other products.
This product will be delivered within 1-3 business days.
Table of Contents
Executive Summary
Adalimumab, Infliximab and Etanercept Biosimilars Global Market Report 2025 provides strategists, marketers and senior management with the critical information they need to assess the market.This report focuses on adalimumab, infliximab and etanercept biosimilars market which is experiencing strong growth. The report gives a guide to the trends which will be shaping the market over the next ten years and beyond.
Reasons to Purchase:
- Gain a truly global perspective with the most comprehensive report available on this market covering 15 geographies.
- Assess the impact of key macro factors such as geopolitical conflicts, trade policies and tariffs, post-pandemic supply chain realignment, inflation and interest rate fluctuations, and evolving regulatory landscapes.
- Create regional and country strategies on the basis of local data and analysis.
- Identify growth segments for investment.
- Outperform competitors using forecast data and the drivers and trends shaping the market.
- Understand customers based on the latest market shares.
- Benchmark performance against key competitors.
- Suitable for supporting your internal and external presentations with reliable high quality data and analysis
- Report will be updated with the latest data and delivered to you along with an Excel data sheet for easy data extraction and analysis.
- All data from the report will also be delivered in an excel dashboard format.
Description
Where is the largest and fastest growing market for adalimumab, infliximab and etanercept biosimilars? How does the market relate to the overall economy, demography and other similar markets? What forces will shape the market going forward, including technological disruption, regulatory shifts, and changing consumer preferences? The adalimumab, infliximab and etanercept biosimilars market global report answers all these questions and many more.The report covers market characteristics, size and growth, segmentation, regional and country breakdowns, competitive landscape, market shares, trends and strategies for this market. It traces the market’s historic and forecast market growth by geography.
- The market characteristics section of the report defines and explains the market.
- The market size section gives the market size ($b) covering both the historic growth of the market, and forecasting its development.
- The forecasts are made after considering the major factors currently impacting the market. These include: the technological advancements such as AI and automation, Russia-Ukraine war, trade tariffs (government-imposed import/export duties), elevated inflation and interest rates.
- Market segmentations break down the market into sub markets.
- The regional and country breakdowns section gives an analysis of the market in each geography and the size of the market by geography and compares their historic and forecast growth.
- The competitive landscape chapter gives a description of the competitive nature of the market, market shares, and a description of the leading companies. Key financial deals which have shaped the market in recent years are identified.
- The trends and strategies section analyses the shape of the market as it emerges from the crisis and suggests how companies can grow as the market recovers.
Scope
Markets Covered:
1) By Product: Adalimumab Biosimilars, Infliximab Biosimilars, Cipleumab2) By Distribution Channel: Hospital Pharmacies, Retail Pharmacies, Online Pharmacies
3) By Application: Crohn’s Disease, Psoriatic Arthritis, Rheumatoid Arthritis, Ulcerative Colitis, Ankylosing Spondylitis, Plaque Psoriasis, Other Applications
Subsegments:
1) By Adalimumab Biosimilars: Amjevita (Amgen); Hyrimoz (Sandoz); Cyltezo (Boehringer Ingelheim); Others2) By Infliximab Biosimilars: Inflectra (Pfizer); Remsima (Celltrion); Ixifi (Pfizer); Others
3) By Cipleumab: Cipleumab Biosimilars; Other Biosimilars in Development
Companies Mentioned: Biogen; Novartis (Sandoz); Pfizer; Amgen; Celltrion; Samsung Bioepis (Samsung Biologics); Hetero Drugs Limited; Fresenius Kabi AG; Boehringer Ingelheim; Biocon; Zydus Lifesciences Limited; Reliance Life Sciences; Torrent Pharmaceuticals Ltd; Sun Pharmaceutical Industries Ltd; Cipla Limited; Zhejiang Hisun Pharmaceutical Co. Ltd; Bio-Thera Solutions, Ltd; Innovent Biologics (Suzhou) Co. Ltd; Janssen Biologics BV; Shanghai CP Guojian Pharmaceutical Co. Ltd; Sansheng Guojian; Shanghai Junshi Biosciences Co. Ltd; Mabwell Bioscience Co. Ltd; LG Chem; Nippon Kayaku; Mochida Pharmaceutical Co. Ltd; mAbxience; Allergan; Microgen; Geropharm; Valenta; NovaMedica Veropharm; Biocad; Roche; Bristol-Myers Squibb; Celon Pharma; SynBio; Ache; Eurofarma; Teva Pharmaceuticals; Oramed Pharmaceuticals; Entera Bio; Julphar; Hikma Pharmaceuticals; Mylan; BIOPHARMA-MEA; NeoTX; AID Genomics Limited; Altis Biologics; Next Biosciences; Viome; Inqaba Biotechnical Industries (Pty) Ltd
Countries: Australia; Brazil; China; France; Germany; India; Indonesia; Japan; Russia; South Korea; UK; USA; Canada; Italy; Spain
Regions: Asia-Pacific; Western Europe; Eastern Europe; North America; South America; Middle East; Africa
Time Series: Five years historic and ten years forecast.
Data: Ratios of market size and growth to related markets, GDP proportions, expenditure per capita.
Data Segmentation: Country and regional historic and forecast data, market share of competitors, market segments.
Sourcing and Referencing: Data and analysis throughout the report is sourced using end notes.
Delivery Format: PDF, Word and Excel Data Dashboard.
Companies Mentioned
The companies featured in this Adalimumab, Infliximab and Etanercept Biosimilars market report include:- Biogen
- Novartis (Sandoz)
- Pfizer
- Amgen
- Celltrion
- Samsung Bioepis (Samsung Biologics)
- Hetero Drugs Limited
- Fresenius Kabi AG
- Boehringer Ingelheim
- Biocon
- Zydus Lifesciences Limited
- Reliance Life Sciences
- Torrent Pharmaceuticals Ltd
- Sun Pharmaceutical Industries Ltd
- Cipla Limited
- Zhejiang Hisun Pharmaceutical Co. Ltd
- Bio-Thera Solutions, Ltd
- Innovent Biologics (Suzhou) Co. Ltd
- Janssen Biologics BV
- Shanghai CP Guojian Pharmaceutical Co. Ltd
- Sansheng Guojian
- Shanghai Junshi Biosciences Co. Ltd
- Mabwell Bioscience Co. Ltd
- LG Chem
- Nippon Kayaku
- Mochida Pharmaceutical Co. Ltd
- mAbxience
- Allergan
- Microgen
- Geropharm
- Valenta
- NovaMedica Veropharm
- Biocad
- Roche
- Bristol-Myers Squibb
- Celon Pharma
- SynBio
- Ache
- Eurofarma
- Teva Pharmaceuticals
- Oramed Pharmaceuticals
- Entera Bio
- Julphar
- Hikma Pharmaceuticals
- Mylan
- BIOPHARMA-MEA
- NeoTX
- AID Genomics Limited
- Altis Biologics
- Next Biosciences
- Viome
- Inqaba Biotechnical Industries (Pty) Ltd
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 250 |
| Published | September 2025 |
| Forecast Period | 2025 - 2029 |
| Estimated Market Value ( USD | $ 4.96 Billion |
| Forecasted Market Value ( USD | $ 7.34 Billion |
| Compound Annual Growth Rate | 10.3% |
| Regions Covered | Global |
| No. of Companies Mentioned | 52 |