Prefilled Syringe Packaging Market
The Prefilled Syringe (PFS) Packaging market sits at the intersection of sterile containment, drug-device combination design, and patient-centric delivery. It primarily serves biologics, vaccines, insulin and GLP-1s, anticoagulants, specialty injectables, and emergency therapeutics where accurate dosing, low extractables/leachables, and robust container-closure integrity are mission-critical. Glass remains the benchmark for dimensional stability and barrier performance, while advanced polymers (COP/COC) are scaling to address break-resistance, low particulate risk, and tungsten-free manufacturing - especially for sensitive biologics and high-viscosity formulations. Ready-to-Use (RTU) nests/tubs, low-silicone or silicone-free systems, coated elastomers, baked-on silicone, and needle-safety accessories are now standard decision points, alongside ISO 11040 compliance and evolving sterile manufacturing requirements (e.g., EU Annex 1) that elevate contamination control strategies and process automation. Regulatory scrutiny over particulates, delamination, and nitrosamine risks has intensified component selection and incoming QC, while Annex 1’s emphasis on CCS and PUPSIT influences upstream partner qualification. Growth is amplified by home-based care, auto-injector compatibility, and expanding fill-finish capacity, with suppliers differentiating through ultra-clean components, RTU flexibility, and device integration. Competitive intensity is high across container manufacturing (glass/polymer), elastomer and coatings, safety systems, and secondary packaging/serialization. Leading players invest in localized capacity, integrated offerings (syringe + elastomer + safety device), and sustainability advances such as solvent-free lubrication, recyclable boards, and reduced plastics. As combination-product expectations climb, successful vendors orchestrate materials science, process capability, and regulatory know-how to deliver consistent performance from aseptic line to patient use.Prefilled Syringe Packaging Market Key Insights
- RTU dominance and flexibility. RTU nests/tubs shorten tech-transfer and enable multi-format, small-lot biologics with faster line changeovers. Converters differentiate via ultra-low particulate profiles and pre-validated sterilization. Pharmaceutical buyers prize short lead times and validated sterility assurance levels. RTU platforms increasingly standardize tub footprints for multi-OEM compatibility. The net effect is de-risked scale-up and better COGS predictability for high-mix portfolios.
- Glass vs polymer trade-offs. Type I glass remains preferred for barrier and dimensional control, but is challenged by breakage, delamination, and tungsten residues. COP/COC offer clarity, impact resistance, and tungsten-free molding for sensitive biologics. Polymer adoption rises with higher volumes and larger formats (e.g., 2.25-5 mL) for viscous drugs. Selection hinges on sorption/oxygen permeability and device fit. Hybrids (glass barrel + polymer components) balance risk and cost.
- Silicone strategy is a differentiator. Free silicone can create subvisible particles and affect biologic stability. Baked-on or immobilized silicone lowers migration while preserving glide force. Some platforms pursue silicone-free plungers and barrier layers to meet ultra-clean specs. Trade-offs include assembly complexity and cost. Robust incoming QC and real-time monitoring of glide force profiles are now expected.
- Elastomer innovation and coatings. Chlorobutyl/bromobutyl plungers with fluoropolymer or film coatings reduce extractables/leachables and protein interactions. Low-metal catalysts, nitrosamine-aware formulations, and tighter cure controls are selection criteria. Vendors position premium SKUs (e.g., ultra-pure, low-particle) for monoclonals and vaccines. Standardization around a few validated SKUs simplifies global submissions and supply assurance.
- Particulate and CCS as purchase drivers. Heightened regulatory focus puts visible/subvisible particulates at the center of audits. Vendors invest in camera inspection, clean-room upgrades, and in-line defect mapping. Robust Container-Closure System (CCS) verification (dye ingress/helium leak surrogates, microbial ingress rationale) is now a must-have. Annex 1 elevates contamination control strategies and PUPSIT, impacting supplier qualification.
- Device integration and human factors. Autoinjector and safety-needle compatibility dictates barrel tolerances, flange geometry, and lubricity windows. Human-factors data favors low activation forces, audible/visual end-of-dose cues, and needle-stick prevention. Suppliers co-develop PFS and device kits to de-risk assembly. Standard platform geometries speed global rollout while allowing brand-specific differentiation.
- Format expansion for viscous biologics. Growth in high-concentration monoclonals and GLP-1 analogs drives 2.25 mL and 3-5 mL PFS interest. Glide-force stability and plunger seal integrity over shelf life become critical. Needle gauge optimization balances pain and flow. Line retrofits (higher torque, adaptive vision) and thermal management for fill-finish are common capex themes.
- Quality analytics and digitalization. Inline vision, SPC dashboards, and defect-signature libraries improve yield and lot-release confidence. Traceability extends from component batch to device assembly and shipper. Real-time trending flags silicone haze, stopper particulates, and flange chips earlier. Digital Device History Records (DHR) support faster deviations closure and global submissions.
- Sustainability without sterility compromise. Brands prioritize solvent-free lubrication, recyclable or fiber-based secondary packaging, and lighter protective components. Life-cycle assessments inform board weight and blister choices. Polymer barrels reduce breakage waste in distribution. The constraint: changes must preserve CCS and transport robustness (ISTA, thermal excursions).
- Supply assurance and regionalization. Dual-sourcing, localized molding/sterilization, and elastic capacity contracts mitigate shocks. Standard RTU SKUs ease interchangeability across sites. Strategic partnerships (container + elastomer + safety device) simplify procurement and tech-transfer. Vendors that pair capacity visibility with technical services (E&L packages, device fit studies) gain preferred-supplier status.
Prefilled Syringe Packaging Market Reginal Analysis
North America
Strong biologics and GLP-1 pipelines, deep device ecosystems, and rigorous combination-product oversight define buyer expectations. Sponsors emphasize ultra-clean RTU components, device integration data, and human-factors validation. Contract fill-finish capacity expansions and serialization maturity support rapid launches. Sustainability goals push solvent-free lubrication and board light-weighting, but CCS remains non-negotiable.Europe
Annex 1 drives contamination control upgrades, barrier isolators, and supplier re-qualification. Preference for needle-safety compliance and standardized RTU tubs aligns with multi-site manufacturing strategies. Polymer adoption grows where tungsten-free pathways aid regulatory comfort for sensitive biologics. Sustainability directives encourage recyclable secondary packs and reduced plastics in transit components.Asia-Pacific
Rapid vaccine and biologics capacity growth in China, India, and Southeast Asia is expanding demand for RTU platforms and localized elastomer supply. Regional CMOs/CDMOs invest in high-speed fill-finish lines and vision inspection. Cost sensitivity favors standardized SKUs, while premium coated elastomers win in export-oriented programs. Governments encourage domestic medical-device value chains and reliability.Middle East & Africa
Market is nascent but advancing via vaccine manufacturing hubs and public-health programs. Procurement focuses on proven RTU systems, robust secondary packaging for climatic stresses, and supplier training for aseptic handling. Partnerships with global vendors and tech-transfer arrangements are common. Donor-funded initiatives emphasize quality documentation and cold-chain resilience.South & Central America
Growth centers on national immunization campaigns, tender-driven insulin/biologic purchases, and expanding CMO footprints. Buyers value container durability through logistics, reliable CCS under temperature excursions, and compliance documentation for audits. Budget constraints favor standardized, validated RTU offerings; vendors that provide tech-service support and rapid remediation gain share.Prefilled Syringe Packaging Market Segmentation
By Type
- Glass Material
- Plastic Material
By Application
- Vaccines
- Antithrombotic Drugs
Key Market players
Becton Dickinson (BD), Gerresheimer, SCHOTT Pharma, Stevanato Group (Ompi), Nipro PharmaPackaging, Terumo, West Pharmaceutical Services, Daikyo Seiko, Datwyler, Aptar Pharma, SHL Medical, Nemera, Ypsomed, Owen Mumford, CatalentPrefilled Syringe Packaging Market Analytics
The report employs rigorous tools, including Porter’s Five Forces, value chain mapping, and scenario-based modelling, to assess supply-demand dynamics. Cross-sector influences from parent, derived, and substitute markets are evaluated to identify risks and opportunities. Trade and pricing analytics provide an up-to-date view of international flows, including leading exporters, importers, and regional price trends.Macroeconomic indicators, policy frameworks such as carbon pricing and energy security strategies, and evolving consumer behaviour are considered in forecasting scenarios. Recent deal flows, partnerships, and technology innovations are incorporated to assess their impact on future market performance.
Prefilled Syringe Packaging Market Competitive Intelligence
The competitive landscape is mapped through proprietary frameworks, profiling leading companies with details on business models, product portfolios, financial performance, and strategic initiatives. Key developments such as mergers & acquisitions, technology collaborations, investment inflows, and regional expansions are analyzed for their competitive impact. The report also identifies emerging players and innovative startups contributing to market disruption.Regional insights highlight the most promising investment destinations, regulatory landscapes, and evolving partnerships across energy and industrial corridors.
Countries Covered
- North America - Prefilled Syringe Packaging market data and outlook to 2034
- United States
- Canada
- Mexico
- Europe - Prefilled Syringe Packaging market data and outlook to 2034
- Germany
- United Kingdom
- France
- Italy
- Spain
- BeNeLux
- Russia
- Sweden
- Asia-Pacific - Prefilled Syringe Packaging market data and outlook to 2034
- China
- Japan
- India
- South Korea
- Australia
- Indonesia
- Malaysia
- Vietnam
- Middle East and Africa - Prefilled Syringe Packaging market data and outlook to 2034
- Saudi Arabia
- South Africa
- Iran
- UAE
- Egypt
- South and Central America - Prefilled Syringe Packaging market data and outlook to 2034
- Brazil
- Argentina
- Chile
- Peru
Research Methodology
This study combines primary inputs from industry experts across the Prefilled Syringe Packaging value chain with secondary data from associations, government publications, trade databases, and company disclosures. Proprietary modeling techniques, including data triangulation, statistical correlation, and scenario planning, are applied to deliver reliable market sizing and forecasting.Key Questions Addressed
- What is the current and forecast market size of the Prefilled Syringe Packaging industry at global, regional, and country levels?
- Which types, applications, and technologies present the highest growth potential?
- How are supply chains adapting to geopolitical and economic shocks?
- What role do policy frameworks, trade flows, and sustainability targets play in shaping demand?
- Who are the leading players, and how are their strategies evolving in the face of global uncertainty?
- Which regional “hotspots” and customer segments will outpace the market, and what go-to-market and partnership models best support entry and expansion?
- Where are the most investable opportunities - across technology roadmaps, sustainability-linked innovation, and M&A - and what is the best segment to invest over the next 3-5 years?
Your Key Takeaways from the Prefilled Syringe Packaging Market Report
- Global Prefilled Syringe Packaging market size and growth projections (CAGR), 2024-2034
- Impact of Russia-Ukraine, Israel-Palestine, and Hamas conflicts on Prefilled Syringe Packaging trade, costs, and supply chains
- Prefilled Syringe Packaging market size, share, and outlook across 5 regions and 27 countries, 2023-2034
- Prefilled Syringe Packaging market size, CAGR, and market share of key products, applications, and end-user verticals, 2023-2034
- Short- and long-term Prefilled Syringe Packaging market trends, drivers, restraints, and opportunities
- Porter’s Five Forces analysis, technological developments, and Prefilled Syringe Packaging supply chain analysis
- Prefilled Syringe Packaging trade analysis, Prefilled Syringe Packaging market price analysis, and Prefilled Syringe Packaging supply/demand dynamics
- Profiles of 5 leading companies - overview, key strategies, financials, and products
- Latest Prefilled Syringe Packaging market news and developments
Additional Support
With the purchase of this report, you will receive:- An updated PDF report and an MS Excel data workbook containing all market tables and figures for easy analysis.
- 7-day post-sale analyst support for clarifications and in-scope supplementary data, ensuring the deliverable aligns precisely with your requirements.
- Complimentary report update to incorporate the latest available data and the impact of recent market developments.
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
- Becton Dickinson (BD)
- Gerresheimer
- SCHOTT Pharma
- Stevanato Group (Ompi)
- Nipro PharmaPackaging
- Terumo
- West Pharmaceutical Services
- Daikyo Seiko
- Datwyler
- Aptar Pharma
- SHL Medical
- Nemera
- Ypsomed
- Owen Mumford
- Catalent
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 160 |
| Published | November 2025 |
| Forecast Period | 2025 - 2034 |
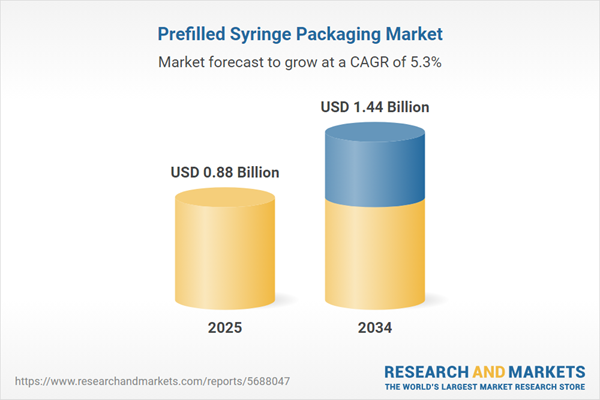
| Estimated Market Value ( USD | $ 0.88 Billion |
| Forecasted Market Value ( USD | $ 1.44 Billion |
| Compound Annual Growth Rate | 5.3% |
| Regions Covered | Global |
| No. of Companies Mentioned | 15 |