Disposable Prefilled Syringes Market
The Disposable Prefilled Syringes (PFS) market sits at the intersection of drug-device combination products, self-administration, and injectable biologics growth. PFS deliver unit-dose accuracy, reduce preparation steps and medication errors, and improve turnaround across hospitals, ambulatory centers, and home care. Core formats include glass and polymer (COC/COP) barrels in standard ISO dimensions, safety-engineered and autoinjector-compatible presentations, staked-needle and luer-lock variants, and RTU/RTC nests and tubs for fill-finish. Top applications span vaccines, anticoagulants, ophthalmics, CNS agents, and high-value biologics in immunology and oncology. Current trends emphasize platform standardization for faster tech transfer, silicone-oil and tungsten management, low-extractable closures, and polymer adoption for break-resistance and low-temperature stability. Drug owners increasingly seek container-closure integrity under cold chain and deep-cold conditions, tight particulate control, and long-dated shelf life. On operations, investments focus on high-speed isolator lines, nested RTU supply, advanced in-process controls, 100% visual inspection, and digital traceability. Competitive dynamics blend global containment specialists, elastomer and closure suppliers, and CMOs/CDMOs providing integrated drug-product services. Differentiation centers on material science (glass quality, polymer clarity), elastomer coatings and barrier films, plunger glide control, device integration readiness, and regulatory/quality track record. Headwinds include capacity bottlenecks during vaccine surges, supply resilience for glass tubing and elastomers, line changeover complexity, and evolving regulations for extractables/leachables. Overall, suppliers combining robust primary packaging science, high-throughput sterile operations, and device ecosystem partnerships are best positioned as self-injection, home care, and combination-product launches expand.Disposable Prefilled Syringes Market Key Insights
- Shift to self-administration: Home care and specialty pharmacy channels favor PFS and devices that simplify handling, shorten teach time, and improve adherence - driving demand for autoinjector-compatible formats and safety features.
- Glass vs. polymer calculus: Glass remains the reference for chemical compatibility and heritage, while COC/COP polymers win on break resistance, low-temperature robustness, and reduced tungsten risk for sensitive biologics.
- Surface & silicone management: Optimized silicone levels, cross-linked oils, or silicone-free barrel options reduce subvisible particles and aggregation risk, while maintaining acceptable glide forces for device performance.
- Closure and elastomer innovation: Fluoropolymer-laminated and coated plungers/needle shields cut extractables and adsorption, stabilizing protein formulations and enabling longer dating across temperature excursions.
- RTU/RTC standardization: Nested, sterilized syringes and compatible plungers/closures shorten tech transfer and enable multi-site comparability; suppliers expand tub/nest formats aligned to common isolator/filler platforms.
- Cold chain and deep-cold readiness: Materials and seals are validated for refrigerated, frozen, and ultra-cold distribution with proven container-closure integrity, thermal shock tolerance, and label/ink durability.
- Device ecosystem integration: PFS designed for autoinjectors and safety sleeves reduce mechanical tolerance risks; co-development with device makers and shared specifications accelerate launch timelines.
- Quality and inspection rigor: 100% automated inspection, robust cosmetic standards, and particulate control are gating criteria, especially for ophthalmics and biologics where micro-defects drive rejects.
- Regulatory and analytics depth: Comprehensive E&L packages, orthogonal subvisible particle methods, and real-time release strategies de-risk filings and post-approval changes across regions.
- Supply-chain resilience: Dual-sourcing of tubing, elastomers, and RTU components, along with flexible fill-finish capacity and standardized formats, mitigates surge demand and geopolitical disruptions.
Disposable Prefilled Syringes Market Reginal Analysis
North America
Strong biologics pipelines, specialty pharmacy growth, and home-care models sustain high PFS uptake. Buyers prioritize RTU glass and polymer platforms validated for cold chain, robust E&L data, and autoinjector compatibility. Contract manufacturers with isolator-based lines, rapid tech transfer, and device integration support gain share, while hospital systems favor safety-engineered formats to meet needlestick policies.Europe
A mature injectables ecosystem and stringent quality frameworks drive emphasis on extractables/leachables, particulate control, and stability in multi-dose vaccine and biologic programs. Preference for standardized RTU components, coated elastomers, and sustainability reporting is rising. Regional CMOs/CDMOs compete on aseptic isolator capacity, visual inspection accuracy, and proven comparability across sites.Asia-Pacific
Rapid expansion in vaccines, biosimilars, and hospital infrastructure fuels demand. Local suppliers scale RTU components and elastomers, while multinational programs require global comparability and data packages. Cost-sensitive markets adopt glass PFS widely; polymer PFS grow for cold-chain durability and break resistance. Training on device use and pharmacovigilance strengthens post-launch outcomes.Middle East & Africa
Procurement programs for immunization, diabetes, and specialty injectables increasingly specify PFS for dosing accuracy and safety. Priorities include robust cold-chain performance, tamper-evident features, and reliable supply with technical support. Partnerships for local fill-finish and technology transfer are emerging alongside investments in clinician and patient training.South & Central America
Public health initiatives and expanding private care systems add steady PFS demand for vaccines and chronic therapies. Buyers look for dependable RTU supply, clear E&L documentation, and packaging suited to variable logistics. Regional CDMOs and distributors with cold-chain know-how and device training capabilities are well positioned to support rollouts and lifecycle management.Disposable Prefilled Syringes Market Segmentation
By Material
- Glass Syringes
- Plastic Syringes
By Application
- Vaccines and immunizations
- Anaphylaxis
- Rheumatoid Arthritis
- Diabetes
- Autoimmune diseases
- Oncology
- Others
By Distribution Channel
- Hospitals
- Retail Pharmacies
- Online Pharmacies
Key Market players
BD (Becton Dickinson), Gerresheimer AG, SCHOTT AG, West Pharmaceutical Services, Nipro Corporation, Stevanato Group, Catalent Inc, Baxter International, Haselmeier GmbH, Owen Mumford, Weigao Group, Vetter Pharma, Terumo Corporation, SHL Medical, Hindustan Syringes & Medical DevicesDisposable Prefilled Syringes Market Analytics
The report employs rigorous tools, including Porter’s Five Forces, value chain mapping, and scenario-based modelling, to assess supply-demand dynamics. Cross-sector influences from parent, derived, and substitute markets are evaluated to identify risks and opportunities. Trade and pricing analytics provide an up-to-date view of international flows, including leading exporters, importers, and regional price trends.Macroeconomic indicators, policy frameworks such as carbon pricing and energy security strategies, and evolving consumer behaviour are considered in forecasting scenarios. Recent deal flows, partnerships, and technology innovations are incorporated to assess their impact on future market performance.
Disposable Prefilled Syringes Market Competitive Intelligence
The competitive landscape is mapped through proprietary frameworks, profiling leading companies with details on business models, product portfolios, financial performance, and strategic initiatives. Key developments such as mergers & acquisitions, technology collaborations, investment inflows, and regional expansions are analyzed for their competitive impact. The report also identifies emerging players and innovative startups contributing to market disruption.Regional insights highlight the most promising investment destinations, regulatory landscapes, and evolving partnerships across energy and industrial corridors.
Countries Covered
- North America - Disposable Prefilled Syringes market data and outlook to 2034
- United States
- Canada
- Mexico
- Europe - Disposable Prefilled Syringes market data and outlook to 2034
- Germany
- United Kingdom
- France
- Italy
- Spain
- BeNeLux
- Russia
- Sweden
- Asia-Pacific - Disposable Prefilled Syringes market data and outlook to 2034
- China
- Japan
- India
- South Korea
- Australia
- Indonesia
- Malaysia
- Vietnam
- Middle East and Africa - Disposable Prefilled Syringes market data and outlook to 2034
- Saudi Arabia
- South Africa
- Iran
- UAE
- Egypt
- South and Central America - Disposable Prefilled Syringes market data and outlook to 2034
- Brazil
- Argentina
- Chile
- Peru
Research Methodology
This study combines primary inputs from industry experts across the Disposable Prefilled Syringes value chain with secondary data from associations, government publications, trade databases, and company disclosures. Proprietary modeling techniques, including data triangulation, statistical correlation, and scenario planning, are applied to deliver reliable market sizing and forecasting.Key Questions Addressed
- What is the current and forecast market size of the Disposable Prefilled Syringes industry at global, regional, and country levels?
- Which types, applications, and technologies present the highest growth potential?
- How are supply chains adapting to geopolitical and economic shocks?
- What role do policy frameworks, trade flows, and sustainability targets play in shaping demand?
- Who are the leading players, and how are their strategies evolving in the face of global uncertainty?
- Which regional “hotspots” and customer segments will outpace the market, and what go-to-market and partnership models best support entry and expansion?
- Where are the most investable opportunities - across technology roadmaps, sustainability-linked innovation, and M&A - and what is the best segment to invest over the next 3-5 years?
Your Key Takeaways from the Disposable Prefilled Syringes Market Report
- Global Disposable Prefilled Syringes market size and growth projections (CAGR), 2024-2034
- Impact of Russia-Ukraine, Israel-Palestine, and Hamas conflicts on Disposable Prefilled Syringes trade, costs, and supply chains
- Disposable Prefilled Syringes market size, share, and outlook across 5 regions and 27 countries, 2023-2034
- Disposable Prefilled Syringes market size, CAGR, and market share of key products, applications, and end-user verticals, 2023-2034
- Short- and long-term Disposable Prefilled Syringes market trends, drivers, restraints, and opportunities
- Porter’s Five Forces analysis, technological developments, and Disposable Prefilled Syringes supply chain analysis
- Disposable Prefilled Syringes trade analysis, Disposable Prefilled Syringes market price analysis, and Disposable Prefilled Syringes supply/demand dynamics
- Profiles of 5 leading companies - overview, key strategies, financials, and products
- Latest Disposable Prefilled Syringes market news and developments
Additional Support
With the purchase of this report, you will receive:- An updated PDF report and an MS Excel data workbook containing all market tables and figures for easy analysis.
- 7-day post-sale analyst support for clarifications and in-scope supplementary data, ensuring the deliverable aligns precisely with your requirements.
- Complimentary report update to incorporate the latest available data and the impact of recent market developments.
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
- BD (Becton Dickinson)
- Gerresheimer AG
- SCHOTT AG
- West Pharmaceutical Services
- Nipro Corporation
- Stevanato Group
- Catalent Inc.
- Baxter International
- Haselmeier GmbH
- Owen Mumford
- Weigao Group
- Vetter Pharma
- Terumo Corporation
- SHL Medical
- Hindustan Syringes & Medical Devices
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 160 |
| Published | November 2025 |
| Forecast Period | 2025 - 2034 |
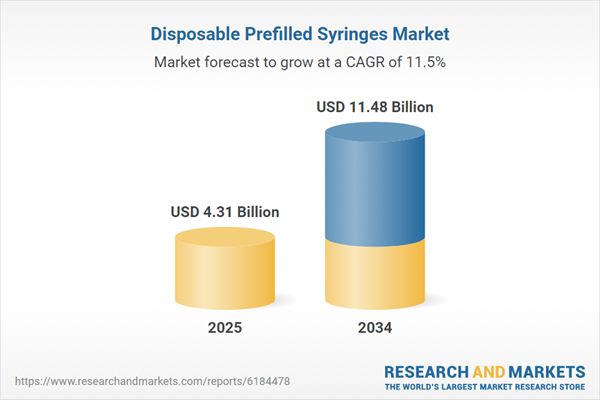
| Estimated Market Value ( USD | $ 4.31 Billion |
| Forecasted Market Value ( USD | $ 11.48 Billion |
| Compound Annual Growth Rate | 11.5% |
| Regions Covered | Global |
| No. of Companies Mentioned | 15 |