Speak directly to the analyst to clarify any post sales queries you may have.
MARKET TRENDS & DRIVERS
Increasing Usage of Advanced Sensors & Artificial Intelligence in Wearable ECG Devices
a) Artificial intelligence (AI) and wearable sensors are two rapidly evolving healthcare technologies poised to transform cardiac care. Together, these technologies enable high-quality electrocardiogram (ECG) acquisition and automatic signal interpretation with cardiologist-level accuracy. Various arrhythmias, such as atrial fibrillation (AF), are detectable, and their diagnosis has important therapeutic implications and may improve patient outcomes.b) Mobile ECG sensors are integrated into wearable devices like smartwatches (Apple Watch, AliveCor Kardia), patches (iRhythm Zio), bands (Fitbit Sense), necklaces (toSense CoVa), clothing, rings, and other wearable forms. Key considerations for device selection include electrode type and configuration, storage capacity, power consumption, battery life, signal processing electronics, and wireless communication capabilities. Most mobile RM devices use a 1-lead ECG configuration for ease of use, but six or more lead configurations (such as the AliveCor KardiaMobile 6L) are also available. The emergence of advanced sensors, AI, ML, and DL in wearable ECG is boosting the wearable ECG devices market.
The Emergence Of Smartwatches Equipped With ECG
a) One of the biggest health features in smartwatches today is the ECG feature. EKG stands for Electrocardiogram and is sometimes abbreviated as EKG. A medical procedure that measures heart activity to check for abnormalities. An electrocardiogram taken in a hospital would require a more detailed reading. Still, the advantage of equipping smartwatches with similar hardware is that the owner can take an electrocardiogram at any time and provide that data to the doctor. The Apple Watch (Series 4, 5, and 6) features an FDA-cleared ECG app that captures 30-second electrocardiogram segments and then analyzes normal rhythm and AF using the user's smartphone.b) Similarly, the Fitbit Sense is another smartwatch-type device with FDA clearance for AF detection. The Samsung Galaxy Watch3 and Galaxy Watch Active2 also include an FDA-cleared ECG monitoring function that includes screening for AF. With the help of built-in connectivity technologies and apps, smartwatches frequently combine ECG data with monitoring other physiologic indicators. They also feature streamlined data storage and transmission capabilities. Such integration of ECG with smartwatches contributes to the growth of the wearable ECG devices market.
The Paradigm Shift from Conventional ECG Towards Ambulatory ECG
a) Ambulatory monitors enable a new paradigm in healthcare by collecting and analyzing long-term data for a reliable diagnosis. These devices are becoming increasingly popular for continuous cardiac monitoring. Advances in hardware and software have created new devices that are convenient and affordable. This allows vulnerable people to be monitored from the comfort of their own homes while providing critical alerts about events requiring urgent medical attention or hospitalization. The essential advantage of wearable ECG devices is that one can continue daily activities while the heart is being monitored.b) Wearable ambulatory monitoring devices, including Holter monitors, are mainly embedded with electrocardiogram sensors for monitoring irregular heartbeats. It is challenging for the HCPs to detect arrhythmic disorders with conventional ECG due to their characteristic of intermittent short episodes. Holter monitors can overcome the challenge faced by conventional ECG devices of low successful diagnosis outcomes. Owing to the benefits of ambulatory monitoring, wearable ECG devices market players are significantly focusing on developing user-friendly, safe & accurate ambulatory ECG devices. For instance, introducing the latest-generation patch-based Holter monitors can be worn up to a few weeks instead of 24/48 hours while offering the greatest patient comfort.
SEGMENTATION ANALYSIS
INSIGHT BY PRODUCT
The smartwatch-based ECG accounted for the largest share of 83.31% in the global wearable ECG devices market in 2021 under the product segment. The higher share can be attributed to the biggest health feature in smartwatches today, the ECG feature. Apple, a key player in smartwatch-based ECG, released its first smartwatch in 2018 and received FDA clearance for automatic AF detection. Still, smartwatches from other leading players such as Samsung, Withings, and Alphabet (Fitbit) also take ECG recordings and alert the wearer when AF is detected. The process of acquiring an electrocardiogram, analyzing it to produce an automated diagnosis of AF, and providing options for communicating those results to the wearer's physician is similar across smartwatch makers. In September 2022, Apple launched Apple Watch Series 8 and the new Apple Watch SE. The ECG app can record the user’s heartbeat and rhythm using the pioneering electrical heart sensor on the company’s Watch Series 4, Series 5, Series 6, Series 7, Series 8, or ultra and then check the recording for AF, a form of irregular rhythm.Segmentation By Product
- Smartwatch-Based ECG
- Mobile Cardiac Telemetry
- Holter Monitoring
- Cardiac Event Monitoring
INSIGHTS BY FREQUENCY
The global wearable ECG devices market is segmented into episodic & Adhoc and continuous by frequency segmentation. The global wearable ECG devices market by episodic & Adhoc market is expected to reach USD 22.28 billion by 2027, growing at a CAGR of 19.48% during the forecast period. Ad-hoc (i.e., one-time) fixed monitoring has been used in many research studies to address different medical settings using different types of ECG sensors and monitoring platforms. Several investigators emphasized that ad hoc non-ambulatory monitoring may reduce the burden of post-ablation outpatient visits and atrial fibrillation-related hospital visits. As the smartwatch-based ECG is the leading segment and has the largest share, 83.31%, in the global wearable ECG devices market, the episodic & ad hoc segment is also the largest share, 62.36% in the global wearable ECG devices market.Segmentation By Frequency
- Episodic & Adhoc
- Continuous
GEOGRAPHICAL ANALYSIS
a) North America accounted for the largest share of 34.08% in the global wearable ECG devices market in 2021 and is likely to witness the highest incremental growth of around USD 9.01 billion during the forecast period. The US is the major revenue contributor to the North American wearable ECG devices market and accounted for a share of 91.30% in 2021. The growth in the region is mainly attributed to the increasing incidence of AF, CVDs such as CAD, surge in diagnosis procedures for treating such diseases. The availability of advanced wearable ECG devices developers, constant technological innovations, and growing acceptance of technologically advanced wearable ECG devices further accelerate the region's market growth.b) The wearable ECG devices market in APAC is growing significantly and is expected to grow at a similar pace during the forecast period. Some factors contributing to this region’s growth are a large pool of patient population, improvements in healthcare infrastructure, and increased healthcare expenditure. Further, the increasing prevalence of cardiac diseases, including AF, the aging population, and the smart wearable adoption trends among the young generation are the key factors driving the wearable ECG devices market in the region.
c) In 2021, Europe accounted for 25.09% of the global wearable ECG devices market share. The rising awareness of ambulatory ECG devices for AF, and CVDs, coupled with improved healthcare infrastructure, various favorable guidelines, and the availability of technologically advanced ECG-based smartwatches, patch are driving the growth of the wearable ECG devices market in the region. Germany, France, the UK, Italy, and Spain are the major revenue generators in the region. These five countries of the ECG devices market collectively accounted for around 65.03% of the European wearable ECG devices market in 2021.
By Geography
- APAC
- China
- India
- Japan
- South Korea
- Australia
- Europe
- Germany
- Italy
- Spain
- France
- UK
- North America
- US
- Canada
- Latin America
- Brazil
- Mexico
- Argentina
- Middle East & Africa
- Turkey
- South Africa
- South Arabia
VENDOR LANDSCAPE
Vendors in the global wearable ECG device market are actively researching and commercializing their novel and advanced wearable ECG device. In the past five years, many vendors received regulatory approvals for their wearable ECG devices. For instance, Withings has received US FDA clearance for the launch of the ScanWatch in the US. The watch was launched in Europe and Australia in 2020 and released in the US in November 2021. In November 2020, VitalConnect, a leader in remote and in-hospital wearable biosensor technology companies, announced that it had launched its VitalPatch RTM cardiac monitoring solution for users who want to expand Holter monitoring. Vendors operating the wearable ECG device market are pursuing various strategies to provide the impetus for growth over the next few years. These market participants adopt collaboration, partnerships, mergers, acquisitions, product launches, and other strategies.Recent Developments In The Global Wearable ECG Devices Market
- In September 2022, Apple launched Apple Watch Series 8 and the new Apple Watch SE.
- In April 2022, Biotricity, a medical diagnostic and healthcare technology player, launched the commercial sales of the Biotres, an FDA-approved wireless wearable extended Holter monitor for Up to 30-day wear duration.
- In 2021, Philips acquired BioTelemetry, a leading vendor in the mobile cardiac telemetry segment.
- In 2021, Boston Scientific acquired Preventice Solutions.
- In December 2021, Baxter International acquired Hill-Rom Services.
ECG-Based Smartwatches: Key Company Profiles
- Apple
- Alphabet
- Samsung Electronics
- Withings
Other Wearable ECG Devices: Key Company Profiles
- Boston Scientific
- GE Healthcare
- iRhythm Technologies
- Hill-Rom
- Koninklijke Philips
- OSI Systems
Other Prominent Vendors
- BPL Medical Technologies
- Bardy Diagnostics
- Bittium
- CardiacSense
- Cortrium
- FUKUDA DENSHI
- Masimo
- Medicalgorithmics
- Advanced Instrumentations
- AMEDTEC MEDIZINTECHNIK AUE
- ASPEL
- Hangzhou Beneware Medical Equipment
- Biotricity
- Borsam Biomedical Instruments
- BTL
- Contec Medical System
- Custo Med
- Oy Diagnostic Devices Development - DDD
- DMS-Service
- EB-Neuro
- Edan Instruments
- Forest Medical
- Holter Supplies
- Labtech
- LPU Medical
- LIVEWELL
- Lumed
- medical ECONET
- Medicomp
- Meditech KFT
- Meditech Equipment
- Midmark
- Monitor
- Nasan Medical Electronics
- Nasiff Associates
- Neurosoft
- Norav Medical
- Northeast Monitoring
- Recorders & Medicare Systems
- Scottcare Cardiovascular Solutions
- Schiller
- Shenzhen Biocare Bio-Medical Equipment
- Suzuken Company
- Trimpeks
- UPOLife
- VivaLNK
KEY QUESTIONS ANSWERED
1. How big is the wearable ECG devices market?2. What is the growth rate of the global wearable ECG devices market?
3. Which region holds the most prominent global wearable ECG devices market share?
4. Who are the global wearable ECG devices market's key players?
5. What are the growth opportunities in the wearable ECG devices market?
Table of Contents
1 Research Methodology2 Research Objectives
3 Research Process
4 Scope & Coverage
4.1 Market Definition
4.1.1 Inclusions
4.1.2 Exclusions
4.1.3 Market Estimation Caveats
4.2 Base Year
4.3 Scope of the Study
4.3.1 Market Segmentation by Geography
5 Report Assumptions & Caveats
5.1 Key Caveats
5.2 Currency Conversion
5.3 Market Derivation
6 Market at a Glance
7 Introduction
7.1 Overview
7.1.1 Wearable Ecg Devices
8 Premium Insights
8.1 Market Scenario
8.1.1 Overview
8.1.2 Market Segmentation
9 Market Opportunities & Trends
9.1 Increasing Use of Advanced Sensors & Ai in Wearable Ecg Devices
9.2 Emergence of Smartwatches Equipped With Ecg
9.3 New Product Approvals & Launches in Wearable Ecg Devices
9.4 Growing Popularity of Smart Wearable Technology in Ecg
10 Market Growth Enablers
10.1 Increasing Global Prevalence of Cardiovascular Diseases
10.2 Paradigm Shift from Conventional Ecg Toward Ambulatory Ecg
10.3 High Demand for Mct & Next-Generation Holter Monitors
11 Market Growth Restraints
11.1 Limitations & Risks Associated With Wearable Ecg Devices
11.2 High Cost of Wearable Ecg Devices
11.3 Availability of Alternate Cardiac Monitoring Devices
12 Market Landscape
12.1 Market Overview
12.2 Market Size & Forecast
12.2.1 Geography Insights
12.2.2 Product Insights
12.2.3 Frequency Insights
12.3 Five Forces Analysis
12.3.1 Threat of New Entrants
12.3.2 Bargaining Power of Suppliers
12.3.3 Bargaining Power of Buyers
12.3.4 Threat of Substitutes
12.3.5 Competitive Rivalry
13 Product
13.1 Market Snapshot & Growth Engine
13.2 Market Overview
13.3 Smartwatch-Based Ecg
13.3.1 Market Overview
13.3.2 Market Size & Forecast
13.3.3 Market by Geography
13.4 Mobile Cardiac Telemetry
13.4.1 Market Overview
13.4.2 Market Size & Forecast
13.4.3 Market by Geography
13.5 Holter Monitoring
13.5.1 Market Overview
13.5.2 Market Size & Forecast
13.5.3 Market by Geography
13.6 Cardiac Event Monitoring
13.6.1 Market Overview
13.6.2 Market Size & Forecast
13.6.3 Market by Geography
14 Frequency
14.1 Market Snapshot & Growth Engine
14.2 Market Overview
14.3 Episodic & Adhoc
14.3.1 Market Overview
14.3.2 Market Size & Forecast
14.3.3 Market by Geography
14.4 Continuous
14.4.1 Market Overview
14.4.2 Market Size & Forecast
14.4.3 Market by Geography
15 Geography
15.1 Market Snapshot & Growth Engine
15.2 Geographic Overview
16 North America
16.1 Market Overview
16.1.1 Market Size & Forecast
16.1.2 North America: Product Segmentation
16.1.3 North America: Frequency Segmentation
16.2 Key Countries
16.2.1 Us: Market Size & Forecast
16.2.2 Canada: Market Size & Forecast
17 Europe
17.1 Market Overview
17.1.1 Market Size & Forecast
17.1.2 Europe: Product Segmentation
17.1.3 Europe: Frequency Segmentation
17.2 Key Countries
17.2.1 Germany: Market Size & Forecast
17.2.2 France: Market Size & Forecast
17.2.3 Uk: Market Size & Forecast
17.2.4 Italy: Market Size & Forecast
17.2.5 Spain: Market Size & Forecast
18 Apac
18.1 Market Overview
18.1.1 Market Size & Forecast
18.1.2 Apac: Product Segmentation
18.1.3 Apac: Frequency Segmentation
18.2 Key Countries
18.2.1 Japan: Market Size & Forecast
18.2.2 China: Market Size & Forecast
18.2.3 India: Market Size & Forecast
18.2.4 South Korea: Market Size & Forecast
18.2.5 Australia: Market Size & Forecast
19 Latin America
19.1 Market Overview
19.1.1 Market Size & Forecast
19.1.2 Latin America: Product Segmentation
19.1.3 Latin America: Frequency Segmentation
19.2 Key Countries
19.2.1 Brazil: Market Size & Forecast
19.2.2 Mexico: Market Size & Forecast
19.2.3 Argentina: Market Size & Forecast
20 Middle East & Africa
20.1 Market Overview
20.1.1 Market Size & Forecast
20.1.2 Middle East & Africa: Product Segmentation
20.1.3 Middle East & Africa: Frequency Segmentation
20.2 Key Countries
20.2.1 Turkey: Market Size & Forecast
20.2.2 Saudi Arabia: Market Size & Forecast
20.2.3 South Africa: Market Size & Forecast
21 Competitive Landscape
21.1 Competition Overview
21.2 Market Share Analysis
22 Ecg-Based Smartwatches: Key Company Profiles
22.1 Apple
22.1.1 Business Overview
22.1.2 Apple in Wearable Ecg Devices Market
22.1.3 Product Offerings
22.1.4 Key Strategies
22.1.5 Key Strengths
22.1.6 Key Opportunities
22.2 Alphabet
22.2.1 Business Overview
22.2.2 Alphabet in Wearable Ecg Devices Market
22.2.3 Product Offerings
22.2.4 Key Strategies
22.2.5 Key Strengths
22.2.6 Key Opportunities
22.3 Samsung Electronics
22.3.1 Business Overview
22.3.2 Samsung Electronics in Wearable Ecg Devices Market
22.3.3 Product Offerings
22.3.4 Key Strategies
22.3.5 Key Strengths
22.3.6 Key Opportunities
22.4 Withings
22.4.1 Business Overview
22.4.2 Withings in Wearable Ecg Devices Market
22.4.3 Product Offerings
22.4.4 Key Strategies
22.4.5 Key Strengths
22.4.6 Key Opportunities
23 Other Wearable Ecg Devices: Key Company Profiles
23.1 Boston Scientific
23.1.1 Business Overview
23.1.2 Boston Scientific in Wearable Ecg Devices Market
23.1.3 Product Offerings
23.1.4 Key Strategies
23.1.5 Key Strengths
23.1.6 Key Opportunities
23.2 GE Healthcare
23.2.1 Business Overview
23.2.2 GE Healthcare in Wearable Ecg Devices Market
23.2.3 Product Offerings
23.2.4 Key Strategies
23.2.5 Key Strengths
23.2.6 Key Opportunities
23.3 Irhythm Technologies
23.3.1 Business Overview
23.3.2 Irhythm Technologies in Wearable Ecg Devices Market
23.3.3 Product Offerings
23.3.4 Key Strategies
23.3.5 Key Strengths
23.3.6 Key Opportunities
23.4 Hill-Rom
23.4.1 Business Overview
23.4.2 Hill-Rom in Wearable Ecg Devices Market
23.4.3 Product Offerings
23.4.4 Key Strategies
23.4.5 Key Strengths
23.4.6 Key Opportunities
23.5 Koninklijke Philips
23.5.1 Business Overview
23.5.2 Koninklijke Philips in Wearable Ecg Devices Market
23.5.3 Product Offerings
23.5.4 Key Strategies
23.5.5 Key Strengths
23.5.6 Key Opportunities
23.6 Osi Systems
23.6.1 Business Overview
23.6.2 Osi Systems in Wearable Ecg Devices Market
23.6.3 Product Offerings
23.6.4 Key Strategies
23.6.5 Key Strengths
23.6.6 Key Opportunities
24 Other Prominent Vendors
24.1 Aerotel Medical Systems
24.1.1 Business Overview
24.1.2 Product Offerings
24.2 Advanced Instrumentations
24.2.1 Business Overview
24.2.2 Product Offerings
24.3 Amedtec Medizintechnik Aue
24.3.1 Business Overview
24.3.2 Product Offerings
24.4 Aspel
24.4.1 Business Overview
24.4.2 Product Offerings
24.5 Bpl Medical Technologies
24.5.1 Business Overview
24.5.2 Product Offerings
24.6 Bardy Diagnostics
24.6.1 Business Overview
24.6.2 Product Offerings
24.7 Bittium
24.7.1 Business Overview
24.7.2 Product Offerings
24.8 Biotricity
24.8.1 Business Overview
24.8.2 Product Offerings
24.9 Borsam Biomedical Instruments
24.9.1 Business Overview
24.9.2 Product Offerings
24.10 Btl
24.10.1 Business Overview
24.10.2 Product Offerings
24.11 Cardiacsense
24.11.1 Business Overview
24.11.2 Product Offerings
24.12 Cortrium
24.12.1 Business Overview
24.12.2 Product Offerings
24.13 Contec Medical Systems
24.13.1 Business Overview
24.13.2 Product Offerings
24.14 Custo Med
24.14.1 Business Overview
24.14.2 Product Offerings
24.15 Dms-Service
24.15.1 Business Overview
24.15.2 Product Offerings
24.16 Eb Neuro
24.16.1 Business Overview
24.16.2 Product Offerings
24.17 Edan Instruments
24.17.1 Business Overview
24.17.2 Product Offerings
24.18 Fukuda Denshi
24.18.1 Business Overview
24.18.2 Product Offerings
24.19 Forest Medical
24.19.1 Business Overview
24.19.2 Product Offerings
24.20 Hangzhou Beneware Medical Equipment
24.20.1 Business Overview
24.20.2 Product Offerings
24.21 Holter Supplies
24.21.1 Business Overview
24.21.2 Product Offerings
24.22 Labtech
24.22.1 Business Overview
24.22.2 Product Offerings
24.23 Lepu Medical Technology
24.23.1 Product Offerings
24.24 Livewell
24.24.1 Business Overview
24.24.2 Product Offerings
24.25 Lumed
24.25.1 Business Overview
24.25.2 Product Offerings
24.26 Masimo
24.26.1 Business Overview
24.26.2 Product Offerings
24.27 Medicalgorithmics
24.27.1 Business Overview
24.27.2 Product Offerings
24.28 Medical Econet
24.28.1 Business Overview
24.28.2 Product Offerings
24.29 Medicomp
24.29.1 Business Overview
24.29.2 Product Offerings
24.30 Meditech Kft
24.30.1 Business Overview
24.30.2 Product Offerings
24.31 Meditech Equipment
24.31.1 Business Overview
24.31.2 Product Offerings
24.32 Midmark
24.32.1 Business Overview
24.32.2 Product Offerings
24.33 Monitor
24.33.1 Business Overview
24.33.2 Product Offerings
24.34 Nasan Medical Electronics
24.34.1 Business Overview
24.34.2 Product Offerings
24.35 Nasiff Associates
24.35.1 Business Overview
24.35.2 Product Offerings
24.36 Neurosoft
24.36.1 Business Overview
24.36.2 Product Offerings
24.37 Norav Medical
24.37.1 Business Overview
24.37.2 Product Offerings
24.38 Northeast Monitoring
24.38.1 Business Overview
24.38.2 Product Offerings
24.39 Oy Diagnostic Devices Development - Ddd
24.39.1 Business Overview
24.39.2 Product Offerings
24.40 Recorders & Medicare Systems
24.40.1 Business Overview
24.40.2 Product Offerings
24.41 Scottcare Cardiovascular Solutions
24.41.1 Business Overview
24.41.2 Product Offerings
24.42 Schiller
24.42.1 Business Overview
24.42.2 Product Offerings
24.43 Shenzhen Biocare Bio-Medical Equipment
24.43.1 Business Overview
24.43.2 Product Offerings
24.44 Suzuken Company
24.44.1 Business Overview
24.44.2 Product Offerings
24.45 Trimpeks
24.45.1 Business Overview
24.45.2 Product Offerings
24.46 Upolife
24.46.1 Business Overview
24.46.2 Product Offerings
24.47 Vivalnk
24.47.1 Business Overview
24.47.2 Product Offerings
25 Report Summary
25.1 Key Takeaways
25.2 Strategic Recommendations
26 Quantitative Summary
26.1 Market by Product
26.1.1 North America: Product Segmentation
26.1.2 Europe: Product Segmentation
26.1.3 Apac: Product Segmentation
26.1.4 Latin America: Product Segmentation
26.1.5 Middle East & Africa: Product Segmentation
26.2 Market by Frequency
26.2.1 North America: Frequency Segmentation
26.2.2 Europe: Frequency Segmentation
26.2.3 Apac: Frequency Segmentation
26.2.4 Latin America: Frequency Segmentation
26.2.5 Middle East & Africa: Frequency Segmentation
26.3 Market by Geography
26.3.1 Smartwatch-Based Ecg: Geography Segmentation
26.3.2 Mobile Cardiac Telemetry: Geography Segmentation
26.3.3 Holter Monitoring: Geography Segmentation
26.3.4 Cardiac Event Monitoring: Geography Segmentation
26.3.5 Episodic & Adhoc: Geography Segmentation
26.3.6 Continuous: Geography Segmentation
27 Appendix
27.1 Abbreviations
Companies Mentioned
- Apple
- Alphabet
- Samsung Electronics
- Withings
- Boston Scientific
- GE Healthcare
- iRhythm Technologies
- Hill-Rom
- Koninklijke Philips
- OSI Systems
- BPL Medical Technologies
- Bardy Diagnostics
- Bittium
- CardiacSense
- Cortrium
- FUKUDA DENSHI
- Masimo
- Medicalgorithmics
- Advanced Instrumentations
- AMEDTEC MEDIZINTECHNIK AUE
- ASPEL
- Hangzhou Beneware Medical Equipment
- Biotricity
- Borsam Biomedical Instruments
- BTL
- Contec Medical System
- Custo Med
- Oy Diagnostic Devices Development - DDD
- DMS-Service
- EB-Neuro
- Edan Instruments
- Forest Medical
- Holter Supplies
- Labtech
- LPU Medical
- LIVEWELL
- Lumed
- medical ECONET
- Medicomp
- Meditech KFT
- Meditech Equipment
- Midmark
- Monitor
- Nasan Medical Electronics
- Nasiff Associates
- Neurosoft
- Norav Medical
- Northeast Monitoring
- Recorders & Medicare Systems
- Scottcare Cardiovascular Solutions
- Schiller
- Shenzhen Biocare Bio-Medical Equipment
- Suzuken Company
- Trimpeks
- UPOLife
- VivaLNK
Methodology
Our research comprises a mix of primary and secondary research. The secondary research sources that are typically referred to include, but are not limited to, company websites, annual reports, financial reports, company pipeline charts, broker reports, investor presentations and SEC filings, journals and conferences, internal proprietary databases, news articles, press releases, and webcasts specific to the companies operating in any given market.
Primary research involves email interactions with the industry participants across major geographies. The participants who typically take part in such a process include, but are not limited to, CEOs, VPs, business development managers, market intelligence managers, and national sales managers. We primarily rely on internal research work and internal databases that we have populated over the years. We cross-verify our secondary research findings with the primary respondents participating in the study.

LOADING...
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 280 |
| Published | January 2023 |
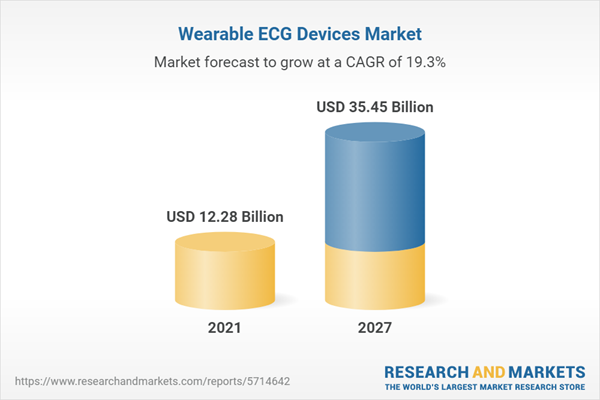
| Forecast Period | 2021 - 2027 |
| Estimated Market Value ( USD | $ 12.28 Billion |
| Forecasted Market Value ( USD | $ 35.45 Billion |
| Compound Annual Growth Rate | 19.3% |
| Regions Covered | Global |
| No. of Companies Mentioned | 56 |