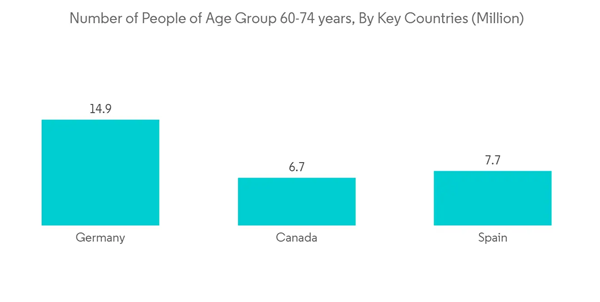The COVID-19 pandemicmoderately impacted the Vitamin D Testing Market in 2020. Stringent lockdown scenarios at home restrictions, postponement of elective procedures, and other restrictions implied by the government during the pandemic resulted in difficulties while accessing healthcare facilities. This subsequently delayed the routing testing procedures, including the vitamin D test. For instance, in a study, “The Impact of COVID-19 on Laboratory Test Utilization at a Pediatric Medical Center”, published by the National Center for Biotechnology Information (NCBI) in September 2022, it was reported that an estimated 84% decrease in lab tests was observed during the pandemic. This was primarily owing to a surge in the lab test volume for the SARS-COV-2 virus, which dramatically offset the diagnostic testing volume for other indications, including the test for vitamin D level.
However, the increasing prevalence of several vitamin D deficiency-associated diseases such as osteoporosis, hair loss, excessive fatigue, and others are augmenting the demand for diagnostic testing of vitamin D. For instance, according to an article published by Informa UK Limited in January 2022, it was reported that globally 8.9 million fractures were reported in each year due to osteoporosis. Also, according to a study published by Harley Street Hair Transplant Clinics in November 2022, it was reported that 15.4 million people in the United Kingdom are suffering from hair loss. These associated disorders are increasing the demand to detect vitamin D levels in the body. This, in turn, is increasing the demand for several vitamin D testing kits globally and fostering the market's growth.
Along with this, the introduction of advanced products for rapid testing and the adoption of several growth strategies adopted by the key players are rising the adoption rate of vitamin D test kits among the common people. For instance, in November 2022, 2San introduced vitamin D rapid test kits for home testing, which offer results within 10 minutes. 2San is one of the leading global manufacturers and distributors of best-in-class medical devices, kits, and infection control systems across the globe. The company has a robust supply chain network in the United Kingdom, Australia, the United States, Canada, and others. Also, in January 2022, MediSure Canada, a subsidiary of Empower Clinics Inc., a telemedicine and medical device company, received the commercialization right from Health Canada for Medisure Vitamin D Rapid Test. This helped the company to cater to the rising demand for vitamin D rapid test kits during the study period. This is augmenting the preference for home test kits and subsequently resulting in the higher adoption of vitamin D tests.
Along with this, rising initiatives for awareness of vitamin D deficiency by the government and private organization is increasing the knowledge of the nutritious value of vitamin D among common people, which in turn increase the rate of vitamin D testing procedures. For instance, in January 2022, Horlicks Women’s Plus partnered with Apollo Clinic to improve bone health awareness among women. In this awareness campaign, they offered free vitamin D tests for women of age group 30 years across Apollo clinics in Chennai, India. Also, the government of England arranged a conference in May 2022 to seek views on improving vitamin D levels among the England population. The main objective of this conference call was to improve population awareness of vitamin D, improving vitamin D levels through diet and dietary supplements. This is alternatively surging the demand and adoption of vitamin D tests among the general population and resulting in the market's overall growth.
Therefore, the aforementioned factors have been instrumental in the substantial growth of the market during the study period. However, certain distinct advantages, including false detection of vitamin D level in the body due to lack of validation of the testing kits, is anticipated to hinder the market growth.
Vitamin D Testing Market Trends
25-Hydroxy Vitamin D Test is Anticipated to Dominate the Market During the Study Period
25-hydroxy vitamin D test is considered the most accurate way to detect the level of vitamin D in a person. According to an article published by Icahn School of Medicine at Mount Sinai in April 2022, it was stated that 25-hydroxy vitamin D test is considered the most precise way to determine the vitamin D level in the body. Also, according to an article published by Healthline Media in January 2022, it was stated that the amount of 25 OH vitamin D is an important indicator to detect osteoporosis and rickets.Additionally, certain benefits, including longer shelf life of 25 OH vitamin D over 1,25 dihydroxy vitamin D, are poised to fuel the adoption of this segment during the study period. For instance, according to an article by NCBI in September 2022, it was reported that owing to the extended half-life of 2 weeks for 25-hydroxy vitamin D, it is mostly recommended for the detection of vitamin D levels in the body as opposed to 1,25 dihydroxy vitamin D, which has a half-life of fewer than 4 hours.
Apart from that, an increasingly sedentary lifestyle decreases exposure to sunlight, and a lower intake of green vegetables and fruits results in a reduced amount of vitamin D in the human body. For instance, according to an article published by HealthifyMe in June 2022, it was stated that several factors, including the primary reason for vitamin D deficiency, is attributed to lack of exposure to sunlight. Also, according to a cross-sectional study conducted in India and published by Informa UK Limited in 2020, it was reported that the prevalence of vitamin D deficiency was found to be around 65.5% among obese patients. This resulted in an increased patient pool suffering from vitamin D deficiency disorders which in turn increased the adoption of the 25-hydroxy vitamin D test during the study period.
In addition to that, a wide range of product availability for the 25-hydroxy vitamin D test is augmenting the adoption rate of this test kit and assay technology among physicians and the general population. For instance, in March 2020, Thermo Fisher Scientific Inc. announced the availability of the Cascadion SM Clinical Analyzer in the United States for 25-hydroxy vitamin D assay testing.
Therefore, the reasons mentioned above are the primary driving factors for the lion’s share of the segment in the market during the study period.
North America is Anticipated to Dominate the Vitamin D Testing Market
North America is expected to dominate the market owing to primary factors, including the rising prevalence of vitamin D deficiency and the rising number of product approvals for vitamin D test kits by the U.S. FDA in the United States. For instance, according to data published by Cleveland Clinic in August 2022, an estimated 35% of adults in the United States suffer from vitamin D deficiency. This augments the demand and adoption of diagnostic kits in this region and alternatively fuels regional growth during the study period.Additionally, to increase awareness of the nutritional value of vitamin D, several organizations are organizing awareness campaigns in the United States. For instance, in August 2020, the Organic & Natural Health Association initiated an educational campaign to enlighten the health benefits of vitamin D. This increased the demand and adoption of vitamin D testing among the general population and subsequently supported the growth of the market in this region.
Apart from that, the availability of favorable reimbursement policies for certain vitamin D tests and increasing awareness among the general population on vitamin D tests is poised to increase the adoption of vitamin D tests in a large proportion. According to UnitedHealthcare Medicare Advantage Policy Guideline in April 2022, it was stated that if the vitamin D level is less than 20 ng/dl or greater than 60 ng/dl, a certain amount of the expense will be reimbursed in the United States.
Therefore, owing to the above-mentioned factors, the North America region is anticipated to lead the regional share during the forecast period.
Vitamin D Testing Market Competitor Analysis
The Vitamin D Testing Market is consolidated in nature due to the presence of a few major players dominating the market shares. The competitive landscape includes an analysis of a few international and local companies that hold market shares and are well known. include F. Hoffmann-La Roche Ltd, DiaSorin S.p.A., Abbott, bioMérieux SA, Siemens Healthcare GmbH, Beckman Coulter, Inc., Quest Diagnostics Incorporated, Quidel Corporation, Thermo Fisher Scientific Inc., Tosoh Bioscience, Inc., Qualigen Therapeutics Inc., among others.Additional benefits of purchasing the report:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
This product will be delivered within 2 business days.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- F. Hoffmann-La Roche Ltd.
- bioMérieux SA
- Beckman Coulter, Inc.
- DiaSorin S.p.A.
- Siemens Healthcare GmbH
- Quest Diagnostics Incorporated
- Abbott
- Quidel Corporation
- Thermo Fisher Scientific Inc.
- Tosoh Bioscience, Inc.
- QUALIGEN THERAPEUTICS, INC.