Speak directly to the analyst to clarify any post sales queries you may have.
10% Free customizationThis report comes with 10% free customization, enabling you to add data that meets your specific business needs.
mRNA Vaccines use a synthetic version of messenger RNA to instruct cells to produce proteins that trigger an immune response. This method enables faster development and manufacturing compared to traditional vaccine platforms. The market is no longer limited to infectious diseases, as ongoing research explores mRNA's application in cancer immunotherapy, genetic disorders, and autoimmune conditions. Advancements in lipid nanoparticle delivery systems have improved the stability and efficiency of mRNA Vaccines, opening new opportunities for therapeutic innovation. Companies are investing heavily in expanding mRNA research pipelines and manufacturing infrastructure to support long-term demand.
Regulatory agencies are also adapting to accommodate the rapid pace of development associated with mRNA technologies. The rise in public awareness and acceptance of mRNA Vaccines has further boosted adoption across various geographies. While challenges such as cold chain logistics and high production costs remain, the market continues to evolve with new entrants and strategic collaborations. As technology matures, mRNA Vaccines are expected to become a cornerstone of modern preventive and therapeutic medicine, offering targeted, personalized, and scalable healthcare solutions.
Key Market Drivers
Rising Demand for Rapid Vaccine Development
The rising demand for rapid vaccine development is a major force driving the global mRNA Vaccine market. For instance, on 29 July 2024, Bio-Manguinhos/Fiocruz, one of Latin America’s largest vaccine manufacturers, joined CEPI’s Global South network. This partnership aims to enhance rapid and equitable vaccine responses to emerging infectious diseases by expanding manufacturing capacity across the Latin American and Caribbean region, strengthening preparedness for future epidemic and pandemic threats through localized production and regional collaboration. Traditional vaccine development can take years, involving complex cultivation, inactivation, or protein purification processes.In contrast, mRNA Vaccines can be designed and produced within weeks once a target pathogen's genetic sequence is known. This speed has become crucial in responding to public health emergencies, as demonstrated during the COVID-19 pandemic. Governments, healthcare organizations, and pharmaceutical companies now recognize the importance of having platforms that allow for quick vaccine adaptation and deployment. mRNA technology offers a flexible and programmable approach. By simply changing the mRNA sequence, developers can target new variants or different diseases without overhauling the entire production process. This flexibility allows for rapid response not only to pandemics but also to emerging infectious threats and seasonal outbreaks. The global health landscape is increasingly shaped by unpredictable disease patterns, making rapid vaccine development a strategic priority.
The ability to accelerate vaccine research and approval timelines also aligns with global efforts to strengthen pandemic preparedness. Investments are being directed toward expanding mRNA production infrastructure, streamlining regulatory pathways, and establishing stockpiles of platform technologies. As demand grows for adaptable and efficient vaccine solutions, mRNA platforms are emerging as essential tools in modern immunization strategies.
Key Market Challenges
Cold chain and storage requirements
Cold chain and storage requirements present a significant challenge to the growth and accessibility of the global mRNA Vaccine market. Unlike traditional vaccines that can often be stored at standard refrigeration temperatures, many first-generation mRNA Vaccines require ultra-cold storage conditions, sometimes as low as -70°C. These extreme requirements create logistical hurdles, especially in developing countries or remote regions where ultra-low temperature freezers and reliable electricity are limited or unavailable. Maintaining the cold chain from manufacturing facilities to administration sites demands specialized equipment, constant temperature monitoring, and trained personnel. Any break in this chain can compromise the integrity and efficacy of the vaccine, leading to waste and reduced immunization rates. This complexity increases distribution costs and slows down deployment, particularly during mass vaccination campaigns or emergency responses.Efforts are being made to develop mRNA Vaccines that are more stable at higher temperatures, which would simplify storage and handling. Some newer formulations have achieved stability at standard refrigeration levels for limited durations, offering hope for wider accessibility. However, such innovations are still under development and not yet broadly available for all mRNA-based products. Until more thermostable solutions become mainstream, the cold chain will remain a critical limiting factor. Addressing this issue is essential for expanding the reach of mRNA Vaccines globally, especially in regions with fragile healthcare infrastructure.
Key Market Trends
Diversification Into Therapeutics Beyond Vaccines
Diversification into therapeutics beyond vaccines is transforming the landscape of the global mRNA Vaccine market. While initial breakthroughs centered on infectious diseases like COVID-19, mRNA technology is now being harnessed for a broad spectrum of therapeutic applications. Oncology is a primary focus, where personalized cancer vaccines use patient-specific tumor mutations to train the immune system to target malignant cells. Companies like BioNTech and Moderna are actively developing clinical programs aimed at various cancers, including melanoma, lung, and pancreatic types.The appeal of mRNA in oncology lies in its adaptability and speed. Developers can rapidly design and produce vaccines that match an individual's cancer profile, offering a level of customization that traditional therapies cannot provide. This approach not only enhances treatment precision but also opens the door to more effective combination therapies with checkpoint inhibitors or immunomodulators. In genetic disorders, mRNA therapies are being explored as a way to replace faulty or missing proteins.
Diseases such as cystic fibrosis or certain metabolic conditions are potential targets, where mRNA could provide temporary yet repeated protein correction. This method offers a less invasive and potentially safer alternative to gene editing technologies. Autoimmune diseases are also emerging as a new frontier. mRNA may help retrain the immune system to tolerate self-antigens, offering novel treatments for conditions like multiple sclerosis or type 1 diabetes. This shift toward broader therapeutic use signals a long-term evolution of the mRNA platform beyond its vaccine origins.
Key Market Players
- Arcturus Therapeutics Holdings Inc.
- BioNTech SE
- CureVac N.V.
- Daiichi Sankyo Company Limited.
- Ethris GmbH
- GlaxoSmithKline plc
- Gennova Biopharmaceuticals Ltd
- Moderna, Inc.
- Pantherna Therapeutics GmbH
- Providence Therapeutics
Report Scope:
In this report, the Global MRNA Vaccine Market has been segmented into the following categories, in addition to the industry trends which have also been detailed below:mRNA Vaccine Market, By mRNA Type:
- Nucleoside-modified mRNA
- Unmodified mRNA
- Self-Amplifying mRNA
mRNA Vaccine Market, By Application:
- COVID-19 mRNA Vaccines
- Non COVID-19 mRNA Vaccines
- Others
mRNA Vaccine Market, By Region:
- North America
- United States
- Canada
- Mexico
- Europe
- France
- United Kingdom
- Italy
- Germany
- Spain
- Asia-Pacific
- China
- India
- Japan
- Australia
- South Korea
- South America
- Brazil
- Argentina
- Colombia
- Middle East & Africa
- South Africa
- Saudi Arabia
- UAE
Competitive Landscape
Company Profiles: Detailed analysis of the major companies present in the Global MRNA Vaccine Market.Available Customizations:
Global MRNA Vaccine Market report with the given Market data, TechSci Research, offers customizations according to a company's specific needs. The following customization options are available for the report.Company Information
- Detailed analysis and profiling of additional Market players (up to five).
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
- Arcturus Therapeutics Holdings Inc.
- BioNTech SE
- CureVac N.V.
- Daiichi Sankyo Company Limited.
- Ethris GmbH
- GlaxoSmithKline plc
- Gennova Biopharmaceuticals Ltd
- Moderna, Inc.
- Pantherna Therapeutics GmbH
- Providence Therapeutics
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 188 |
| Published | August 2025 |
| Forecast Period | 2024 - 2030 |
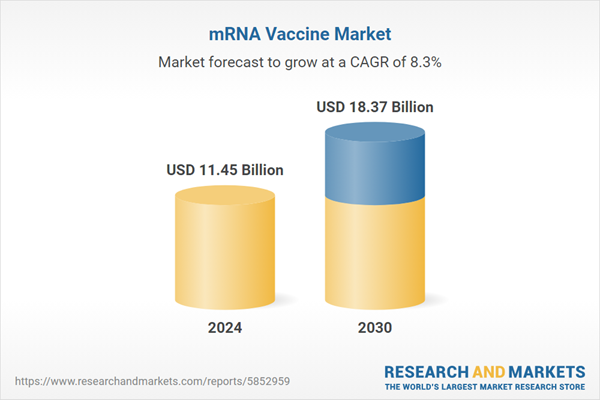
| Estimated Market Value ( USD | $ 11.45 Billion |
| Forecasted Market Value ( USD | $ 18.37 Billion |
| Compound Annual Growth Rate | 8.3% |
| Regions Covered | Global |
| No. of Companies Mentioned | 10 |