Infectious diseases impose a significant economic burden on societies worldwide
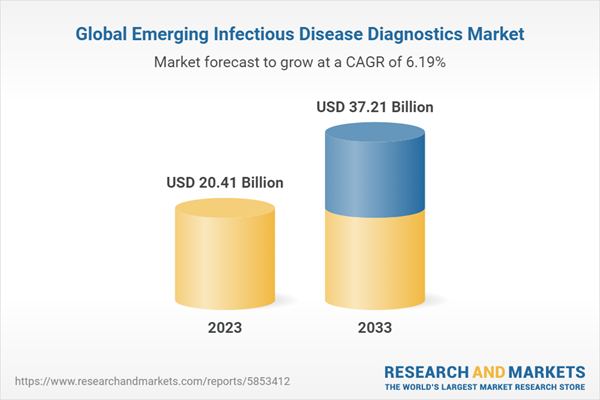
The global emerging infectious disease diagnostics market was valued at $19.56 billion in 2022 and is expected to reach $37.21 billion by 2033, growing at a CAGR of 6.19% between 2023 and 2033. The key factors driving the growth of the global emerging infectious disease diagnostics market include the economic burden of infectious diseases, government support through funding and collaborations, increasing demand for point-of-care and syndromic testing, and technological advancements in molecular diagnostics.
Market Introduction
Infectious disease diagnostics encompass a range of methods and techniques used to identify and detect infectious agents in individuals suspected of having an infection. These diagnostics play a vital role in accurate and timely disease diagnosis, enabling appropriate treatment decisions and public health interventions.
Microbiological culture is a fundamental approach in which patient specimens are cultured on specialized growth media to isolate and identify specific pathogens. This technique allows for the determination of the causative organism and the assessment of its antimicrobial susceptibility. Molecular diagnostics, another important approach, utilizes techniques such as polymerase chain reaction (PCR) and nucleic acid amplification tests (NAATs) to detect the genetic material of the infectious agent. This enables highly sensitive and specific identification of pathogens, even at low concentrations.
Industrial Impact
Infectious disease diagnostics have a significant impact on various aspects of healthcare and public health. Here are some key impacts:
Accurate and Timely Diagnosis: Infectious disease diagnostics enable healthcare providers to accurately identify the causative agents of infections, leading to timely and appropriate treatment. Early diagnosis helps improve patient outcomes, reduce morbidity and mortality rates, and prevent the spread of infectious diseases. Effective Patient Management: Diagnostic tests provide critical information for healthcare providers to guide patient management strategies. They help determine the most effective treatment options, including the selection of appropriate antimicrobial therapies and the implementation of infection control measures. This leads to better patient care and improved healthcare outcomes.
Public Health Surveillance and Outbreak Control: Infectious disease diagnostics play a crucial role in public health surveillance. By identifying and monitoring infectious agents, diagnostic tests contribute to the detection and tracking of disease outbreaks. This information helps public health authorities implement timely interventions, such as contact tracing, isolation measures, and targeted vaccination campaigns, to control the spread of infections.
Antimicrobial Stewardship: Accurate diagnostic tests help in the appropriate use of antimicrobial agents. By identifying the specific pathogens causing infections, healthcare providers can make informed decisions regarding antimicrobial prescriptions, avoiding unnecessary antibiotic use and reducing the risk of antimicrobial resistance.
Research and Development: Infectious disease diagnostics drive research and development efforts to improve existing diagnostic methods and develop new technologies. Ongoing advancements in diagnostics contribute to the discovery of novel biomarkers, the enhancement of diagnostic accuracy and sensitivity, and the integration of innovative approaches such as molecular techniques, biosensors, and artificial intelligence.
Impact of COVID-19
The COVID-19 pandemic has had a profound impact on infectious disease diagnostics globally. The urgent need for widespread testing to identify and control the spread of the virus has led to a rapid expansion of diagnostic capabilities. There has been an unprecedented surge in the development and deployment of diagnostic tests, including nucleic acid-based tests such as PCR and antigen tests, to detect SARS-CoV-2, the virus causing COVID-19. The pandemic has also accelerated advancements in point-of-care testing technologies, such as rapid antigen tests and molecular diagnostics, allowing for faster and more accessible testing.
Furthermore, the pandemic has highlighted the importance of surveillance systems, data sharing, and digital health technologies in monitoring and responding to infectious diseases. The lessons learned from COVID-19 are likely to have a long-lasting impact on the field of infectious disease diagnostics, driving innovations and improvements in testing capabilities, rapid response readiness, and global collaboration.
Market Segmentation
Segmentation 1: by Application
- Laboratory Testing
- Point-of-Care Testing
Point-of-Care Testing to Register Significant Growth during the Forecast Period 2023-2033
Point-of-care (POC) testing for infectious diseases offers rapid and on-site diagnostic capabilities, enabling immediate detection and decision-making. POC tests are designed to be portable, user-friendly, and suitable for use in various healthcare settings. They provide quick results, often within minutes or hours, facilitating timely diagnosis and treatment decisions, particularly crucial for infectious diseases where early intervention can significantly impact patient outcomes and prevent further transmission.
POC tests are particularly useful in developing countries where there is a lack of robust laboratory infrastructure or high-end capital equipment to conduct diagnostic tests. They can help in the timely and accurate diagnosis of diseases in low-resource settings, thereby aiding in relieving the burden of infectious diseases in the region.
Segmentation 2: by Technology
- Polymerase Chain Reaction (PCR)
- Isothermal Nucleic Acid Amplification Technology (INAAT)
- Next-Generation Sequencing (NGS)
- Immunodiagnostics
- Other Technologies
Next-Generation Sequencing (NGS) to Register Maximum Growth in the Market
Next-generation sequencing (NGS) has revolutionized infectious disease diagnosis by enabling comprehensive and high-throughput analysis of pathogen genomes. Whole genome sequencing (WGS) allows the sequencing of entire pathogen genomes, providing detailed information about genetic variations, antimicrobial resistance genes, and virulence factors. It can identify and characterize pathogens, track outbreaks, and analyze their evolutionary patterns.
Other emerging technologies anticipated to register significant growth include technologies such as digital PCR, INAAT, and clustered regularly interspaced short palindromic repeats (CRISPR).
- Digital PCR is an advanced molecular diagnostic technique used for infectious disease diagnostics. It enables the absolute quantification of target nucleic acids by partitioning the PCR reaction into thousands of individual reactions.
- INAAT has emerged as a valuable tool for infectious disease diagnostics. These techniques, such as loop-mediated isothermal amplification (LAMP) and recombinase polymerase amplification (RPA), enable rapid and sensitive detection of pathogens without the need for complex equipment or multiple temperature cycles.
- CRISPR is a relatively new technology, and while it has been leveraged for gene editing and therapeutic purposes ever since the invention of CRISPR/Cas9 gene editing in 2011, it was only in 2016 that CRISPR-Cas systems were first developed to identify nucleic acids for molecular diagnostics.
Segmentation 3: by Type of Infection
- Bacterial
- Viral
- Fungal
- Other Infections
Bacterial and Viral Infections to Dominate the Market
Bacterial infectious disease diagnosis involves various methods and techniques to identify the presence of bacterial pathogens in clinical samples. Some of the common bacterial infections include respiratory infections such as tuberculosis and streptococcal infections and sexually transmitted infections (STIs) such as chlamydia, gonorrhea, and syphilis.
Viruses have the ability to mutate and, therefore, can lead to the re-emergence of diseases even after one form of the virus has been eradicated or managed. Molecular diagnostic companies are therefore focused on developing tests that can detect a wide range of viruses. Some of the common infections caused by viruses include influenza, genital herpes, COVID-19, hepatitis, mumps, rubella, gastroenteritis, Zika disease, and ebola disease, among others.
Segmentation 4: by Disease Type
- Respiratory Infections
- Sexually Transmitted Infections (STIs)
- Gastrointestinal Infections
- Other Infections
Respiratory Infections to Dominate the Market Majorly Driven by the COVID-19 Pandemic
A vast majority of molecular diagnostic companies offer tests for testing various kinds of bacteria and viruses responsible for causing respiratory infections. Moreover, the COVID-19 pandemic led to the development of a wide range of tests employing conventional technologies such as RT-PCR and even leveraging emerging technologies such as RT-LAMP and CRISPR. Several companies now offer multiplex assays that test for influenza A, influenza B, respiratory syncytial virus (RSV), and SARS-CoV-2 virus at once in a single assay.
Segmentation 5: by End User
- Hospitals and Clinics
- Diagnostic Laboratories
- Other End Users
Hospitals and Clinics Continue to Dominate the Market
Hospitals and clinics play a critical role in the field of infectious disease diagnostics, serving as important centers for patient evaluation and testing. In addition to point-of-care testing (POCT), which provides immediate results for rapid diagnosis and management, hospitals and clinics may also rely on laboratory testing for comprehensive analysis. Some healthcare facilities have on-site laboratories equipped with state-of-the-art diagnostic technologies, while others collaborate with external diagnostic laboratories for specialized testing. These laboratory facilities employ various methods, including molecular techniques like PCR and sequencing, serological assays, and culture-based methods to identify and characterize infectious agents accurately.
Segmentation 6: by Region
- North America - U.S., Canada
- Europe - Germany, U.K., France, Italy, Spain, and Rest-of-Europe
- Asia-Pacific - Japan, China, India, South Korea, Australia, Rest-of-Asia-Pacific
- Latin America - Brazil, Mexico, and Rest-of-Latin America
- Middle East and Africa - U.A.E., K.S.A., and Rest-of-Middle East and Africa
North America consists of the U.S. and Canada and accounts for the largest share of the global emerging infectious disease diagnostics market. The growth of the market in North America is driven largely by factors such as the presence of a large number of companies and the presence of robust laboratory infrastructure for conducting molecular diagnostic testing.
Recent Developments in the Emerging Infectious Disease Diagnostics Market
- In June 2023, T2 Biosystems announced a clinical collaboration with Vanderbilt University Medical Center to implement and evaluate the T2 bacteria panel for clinical use.
- In June 2023, T2 biosystems announced submission for FDA breakthrough device designation for its Candida Auris diagnostic test.
- In April 2023, Tecan Trading AG partnered with DYNEX Technologies to expand the availability of ELISA immunoassays in the U.S.
- In April 2023, QIAGEN N.V. introduced new syndromic testing solutions in Japan.
- In February 2023, Thermo Fisher Scientific Inc. and Mylab announced a pact on test kits for infectious diseases.
- In February 2023, Sherlock Biosciences acquired Sense Biodetection, a molecular diagnostic company, to help advance the commercialization of its CRISPR-based diagnostic test.
- In February 2023, Biotech startup CrisprBits raised $250,000 in a pre-seed round to support the development and commercialization of its CRISPR-based tests.
- In February 2023, FDA authorized the Xpert Mpox test by Cepheid (parent: DANAHER CORPORATION) for emergency use.
- In January 2023, CrisprBits developed India’s first CRISPR-based SARS-CoV-2 test with Omicron detection.
Demand - Drivers and Limitations
Market Demand Drivers:
Economic Burden of Infectious Diseases: Infectious diseases impose a significant economic burden on societies worldwide. The direct costs include expenses related to diagnosis, treatment, hospitalization, and healthcare utilization. Indirect costs arise from lost productivity due to illness, disability, or premature death. Furthermore, infectious disease outbreaks can disrupt trade, tourism, and supply chains, impacting sectors such as agriculture, manufacturing, and services.
Government Support to Advance Research and Facilitate Early Diagnosis of Infectious Diseases through Funding and Collaborations: Governments globally have been focused on allocating budgets and providing funding to advance research and early diagnosis in the field of infectious diseases.
Increasing Demand for Point-of-Care (POC) and Syndromic Testing: The demand for point-of-care testing is rising due to its advantages in delivering rapid results and facilitating immediate clinical decision-making. POCT allows for decentralized testing in various healthcare settings, including remote and resource-limited areas, enabling timely diagnosis and treatment initiation. This has driven the adoption of infectious disease diagnostics, particularly in areas with limited laboratory infrastructure.
Technological Advancements in Molecular Diagnostics: Advancements in diagnostic technologies have played a crucial role in the growth of the emerging infectious disease diagnostics market. These advancements include the development of molecular diagnostics, point-of-care testing, immunoassays, and automated systems, among others. These technologies provide rapid and accurate results, enabling healthcare professionals to make timely treatment decisions.
Market Challenges:
Poor Reimbursement for Molecular Diagnostic Tests for Infectious Diseases: The overall gap between reimbursement and cost of point-of-care (POC) testing is increasing. Moreover, in the realm of technology-driven innovations, the endorsement of formal health technology assessments (HTAs) is frequently necessary to facilitate policy-level decisions regarding coverage, reimbursement, and utilization. However, the HTA process itself can be time-consuming and lead to delays in adoption decisions.
Erratic Trend in Infectious Disease Epidemic Outbreaks Globally: The emergence of infectious diseases is a consequence of the interactions between microbes and humans, which often involve complex and multifaceted factors. The erratic trend in the emergence/re-emergence of infectious disease outbreaks can make it very difficult to predict future outbreaks. Moreover, the mutating nature of causative agents such as viruses further complicates predicting the progression of an outbreak and its management.
Lack of an Established Framework for NGS-based Tests for Infectious Diseases: While tests have been made accessible under the EUA framework, the currently established frameworks for NGS-based tests are highly leaning toward oncology, making it inappropriate to be used as a reference for development and commercialization of tests for infectious diseases.
Market Opportunities:
Potential of Molecular Diagnostic Tests in Low-Resource Settings: While molecular diagnostics might not replace culture-based methods in the near future, they offer a significant advantage by providing access to testing for a vast number of individuals. This technology has the potential to revolutionize the surveillance of hard-to-reach populations and efficiently cover a large geographic area with limited resources.
Potential of Rapid Point-of-Care Tests in Managing Antimicrobial Resistance: Rapid diagnostic techniques play a crucial role in identifying newly emerging resistance traits, thereby promoting effective antibiotic stewardship. These techniques are invaluable in addressing two vital clinical questions, i.e., firstly, determining the necessity of antimicrobial therapy for a patient, and secondly, identifying the appropriate antimicrobial for successful treatment in the presence of microbial infection.
How Can This Report Add Value to an Organization?
Product/Innovation Strategy: The global emerging infectious disease diagnostics market has been extensively segmented on the basis of various categories, such as application, technology, type of infection, disease type, end user, and region. This can help readers get a clear overview of which segments account for the largest share and which ones are well-positioned to grow in the coming years.
Growth/Marketing Strategy: Product launches, upgradations, and approvals accounted for the maximum number of key developments, i.e., over 70.00% of the total developments in the global emerging infectious disease diagnostics market, as of June 2023.
Competitive Strategy: The global emerging infectious disease diagnostics market is fragmented, with several established as well as emerging players. Key players in the global emerging infectious disease diagnostics market analyzed and profiled in the study involve established players that offer various kinds of molecular diagnostic tests for infectious diseases.
Key Market Players and Competition Synopsis
The companies that are profiled have been selected based on inputs gathered from primary experts and analyzing company coverage, product portfolio, and market penetration.
The top segment players who are leading include manufacturers offering tests leveraging polymerase chain reaction (PCR) technology, which captured around 42.35% of the presence in the market in 2022. This was followed by immunodiagnostics at 30.75% market share in 2022.
Key Companies Profiled:
- Abbott Laboratories
- Becton, Dickinson and Company
- bioMérieux S.A.
- Bio-Rad Laboratories, Inc.
- Co-Diagnostics, Inc.
- DANAHER CORPORATION
- DiaSorin S.p.A.
- F. Hoffmann-La Roche Ltd
- Hologic, Inc.
- QIAGEN N.V.
- QuidelOrtho Corporation
- T2 Biosystems, Inc.
- Tecan Trading AG
- Thermo Fisher Scientific Inc.
- Siemens Healthineers AG
Table of Contents
Companies Mentioned
- Abbott Laboratories
- Becton, Dickinson and Company
- bioMérieux S.A.
- Bio-Rad Laboratories, Inc.
- Co-Diagnostics, Inc.
- DANAHER CORPORATION
- DiaSorin S.p.A.
- F. Hoffmann-La Roche Ltd
- Hologic, Inc.
- QIAGEN N.V.
- QuidelOrtho Corporation
- T2 Biosystems, Inc.
- Tecan Trading AG
- Thermo Fisher Scientific Inc.
- Siemens Healthineers AG
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 284 |
| Published | July 2023 |
| Forecast Period | 2023 - 2033 |
| Estimated Market Value ( USD | $ 20.41 Billion |
| Forecasted Market Value ( USD | $ 37.21 Billion |
| Compound Annual Growth Rate | 6.2% |
| Regions Covered | Global |
| No. of Companies Mentioned | 15 |