Speak directly to the analyst to clarify any post sales queries you may have.
10% Free customizationThis report comes with 10% free customization, enabling you to add data that meets your specific business needs.
The cell and gene therapy cold chain logistics market in the United Kingdom has been experiencing significant growth due to the increasing number of clinical trials and approvals for cell and gene therapies. As of 6th May 2023 there are around 23,372 clinical trials in different phases of development across the United Kingdom. The United Kingdom has been a pioneer in this field, with the country being the first to approve a gene therapy for a genetic disease in 2012. The growth of the cell and gene therapy market has created a need for specialized logistics services to maintain the integrity of these complex therapies during transportation. Cold chain logistics is crucial for the safe and effective delivery of cell and gene therapies, as they require specific temperature and humidity conditions to maintain their stability and potency.
There are several companies operating in the United Kingdom that specialize in cold chain logistics for cell and gene therapies, including DHL, World Courier, and Biocair. These companies offer a range of services, including temperature-controlled packaging, transport, storage, and monitoring. In 2021, the United Kingdom government also announced a USD 11 million investment to establish a new Cell and Gene Therapy Catapult Innovation Centre in Stevenage, United Kingdom which will provide state-of-the-art facilities for the development and commercialization of advanced therapies. This investment is expected to further drive growth in the cell and gene therapy market and the associated logistics services, thereby supporting the growth of United Kingdom cell & gene therapy cold chain logistics market. Thus, such favorable environment for innovation and growing demand is anticipated to push the United Kingdom cell & gene therapy cold chain logistics market.
Development of Advanced Cold Chain Technologies and Solutions is a Factor that Driving the Market Growth
United Kingdom cell and gene therapy cold chain logistics market is experiencing significant growth, due to the growing demand for cell therapies. Cell therapies involve the use of living cells to treat various diseases, including cancer and genetic disorders. The logistics associated with these therapies are critical to ensure their safe and effective delivery to patients. The demand for cell therapies has been steadily increasing in recent years due to their promising results in clinical trials. In the United Kingdom, there has been a significant increase in the number of clinical trials for cell therapies, which has led to the approval of several cell-based products for commercial use. According to clinicaltrials.gov, there are around 610 studies in different phases of development related to cell therapies in the United Kingdom.The logistics associated with cell therapies are complex and require specialized infrastructure to ensure their safe and efficient transportation. Cold chain logistics is essential for maintaining the viability and potency of these therapies during transportation. This process involves maintaining a specific temperature range throughout the entire supply chain, from production to delivery.
United Kingdom cell and gene therapy cold chain logistics market growth is supported by several companies that specialize in providing these services. These companies offer a range of services, including temperature-controlled packaging, transport, storage, and monitoring. They use advanced technologies to ensure that the cell therapies remain at the required temperature throughout the entire supply chain. The growing demand for cell therapies has also led to increased investment in the United Kingdom's cell and gene therapy sector. The continuous investment in the gene and therapy sector by United Kingdom government can increase its uneven share of the global gene and therapy market to 15% which equates to more than USD 11 billion of revenue (predominately exports) and 18,000 jobs in 2035.
The investment in the United Kingdom cell and gene therapy sector is expected to drive growth in the associated logistics services, including cold chain logistics. As more cell therapies are developed and approved for commercial use, the demand for specialized logistics services will continue to grow. In addition to the growth of the cell and gene therapy sector, the COVID-19 pandemic has also contributed to the increased demand for cold chain logistics services. The pandemic has highlighted the importance of safe and efficient vaccine distribution, which requires specialized logistics services to maintain the integrity of the vaccines during transportation. Thus, all the above-mentioned advancement is expected to drive the growth of the United Kingdom cell & gene therapy cold chain logistics market.
Supportive Regulatory Environment and Government Initiatives is Pushing Cell & Gene Therapy Cold Chain Logistics Market Growth
United Kingdom cell and gene therapy cold chain logistics market is expected to see significant growth in the coming years, on account of the supportive regulatory environment and government initiatives. The United Kingdom has established itself as a global leader in cell and gene therapy research and development, and the government has taken several steps to support the growth of this sector, including initiatives to promote innovation and investment in industry. The United Kingdom has a robust regulatory framework in place that provides a clear path to market for these therapies. The Medicines and Healthcare Products Regulatory Agency (MHRA) is responsible for regulating cell and gene therapies in the United Kingdom, and it has established a dedicated team to support the development and approval of these therapies.The MHRA has also introduced several initiatives to promote the development of cell and gene therapies in the United Kingdom. For instance, United Kingdom has established the Accelerated Access Pathway (AAP), which provides a streamlined regulatory process for innovative therapies that address unmet medical needs. This initiative helps to speed up the development and approval process for cell and gene therapies, enabling them to reach patients more quickly.
The United Kingdom government has also taken several steps to support the growth of the cell and gene therapy industry. In 2018, the government announced the Life Sciences Sector Deal, which included a USD 1.46 billion investment in the sector. This investment is intended to support the development of innovative therapies and technologies, including cell and gene therapies, genomics, and AI-powered diagnosis.
The government has also established the Cell and Gene Therapy Catapult, a center of excellence for the development and commercialization of cell and gene therapies. Catapult provides state-of-the-art facilities and expertise to support the development of these therapies and has helped to position the United Kingdom as a global leader in the industry.
The supportive regulatory environment and government initiatives are expected to propel the growth of the United Kingdom's cell and gene therapy cold chain logistics market. The logistics associated with these therapies are complex and require specialized infrastructure to ensure their safe and efficient transportation. Cold chain logistics is essential for maintaining the viability and potency of these therapies during transportation. As the United Kingdom cell and gene therapy cold chain logistics market are supported by several companies that specialize in providing these services. These companies offer a range of services, including temperature-controlled packaging, transport, storage, and monitoring. They use advanced technologies to ensure that the cell therapies remain at the required temperature throughout the entire supply chain.
Technological Advancements are Key Reason that Responsible for Market Growth
United Kingdom cell and gene therapy cold chain logistics market is continuously evolving due to technological advancements that are transforming the industry. With the increasing demand for cell and gene therapies, it is essential to have efficient and safe logistics processes to transport these therapies. The use of advanced technologies has become a significant trend in the industry, allowing for the optimization of logistics processes and the reduction of risks associated with transportation.The latest trends in the United Kingdom's cell and gene therapy cold chain logistics market are the use of automation and robotics in logistics processes. The use of automated systems and robotics has enabled logistics providers to improve their efficiency and accuracy in handling sensitive and valuable materials. The technology reduces the risk of human error, ensures consistency in processes, and enables faster turnaround times.
The adoption of real-time monitoring and tracking technologies is major trend in the United Kingdom's cell and gene therapy cold chain logistics market. The use of temperature sensors and other monitoring devices enables logistics providers to track the location and temperature of cell and gene therapies throughout the transportation process. The technology helps to ensure that the therapies are transported within the required temperature range, and in case of any deviations, the logistics providers can take corrective actions quickly.
The increasing adoption of cloud-based logistics management systems is also a trend in the United Kingdom's cell and gene therapy cold chain logistics market. These systems provide real-time visibility of logistics processes, allowing logistics providers to monitor the status of the transportation process in real-time. The technology enables efficient collaboration and communication between different stakeholders involved in the logistics processes, including the logistics providers, hospitals, and pharmaceutical companies.
The use of blockchain technology is another key trend in the United Kingdom's cell and gene therapy cold chain logistics market. The technology enables secure and transparent record-keeping of logistics processes. It helps to ensure the authenticity of the transportation process, ensuring that the therapies are not tampered with during transportation. The use of blockchain technology can also help to reduce the risk of counterfeiting and ensure the safety of patients.
The use of artificial intelligence (AI) and machine learning (ML) is also a trend in the United Kingdom's cell and gene therapy cold chain logistics market. The technology enables logistics providers to predict and identify potential transportation issues before they occur, enabling them to take corrective actions proactively. The technology can also help to optimize logistics processes, reducing transportation costs and improving efficiency.
Market Segmentation
United Kingdom Cell & Gene Therapy Cold Chain Logistics market is segmented based on component, services offered, mode sof transportation, holding temperature range, end user, and region. Based on the component, the market is divided into cryogenic shippers, cryogenic storage freezers, ultra-low freezers, cold chain management systems, shipment and storage medium, cryogenic pack out kits, and others {shipment containers, reusable boxes, etc.}. Based on the service offered, the market is divided into transportation, storage, and packaging. Based on the mode of transportation, the market is divided into air, ground, and water. Based on the holding temperature range, the market is divided into cryogenic, refrigerated, ambient, and others {deep freezers, dry ice, etc.}. Based on the end user, the market is divided into pharmaceutical & biotechnology companies, academic & research institutes, and others.Company Profiles
Atelerix Ltd., Life Science Group Ltd (LSG), Arvato Group, DGP Intelius Ltd., QuickSTAT (A Kuehne + Nagel Company), BIOCAIR, TrakCel, Cryoport UK Limited, Patheon UK Limited (Thermo Fischer Scientific UK), Catalent UK are some of the key players of United Kingdom cell & gene therapy cold chain logistics market.Report Scope:
In this report, United Kingdom Cell & Gene Therapy Cold Chain Logistics market has been segmented into the following categories, in addition to the industry trends, which have also been detailed below:United Kingdom Cell & Gene Therapy Cold Chain Logistics Market, By Component:
- Cryogenic Shippers
- Cryogenic Storage Freezers
- Ultra Low Freezers
- Cold Chain Management Systems
- Shipment and Storage Medium
- Cryogenic Packout Kits
- Others include shipment containers, reusable boxes, etc.
United Kingdom Cell & Gene Therapy Cold Chain Logistics Market, By Services Offered:
- Transportation
- Storage
- Packaging
United Kingdom Cell & Gene Therapy Cold Chain Logistics Market, By Mode of Transportation:
- Air
- Ground
- Water
United Kingdom Cell & Gene Therapy Cold Chain Logistics Market, By Holding Temperature Range:
- Cryogenic
- Refrigerated
- Ambient
- Others include deep freezers, dry ice, etc.
United Kingdom Cell & Gene Therapy Cold Chain Logistics Market, By End User:
- Pharmaceutical & Biotechnology Companies
- Academic & Research Institutes
- Others
United Kingdom Cell & Gene Therapy Cold Chain Logistics Market, By Region:
- Scotland
- South-East
- London
- South-West
- East-Anglia
- Yorkshire & Humberside
- East Midlands
Competitive landscape
Company Profiles: Detailed analysis of the major companies in United Kingdom Cell & Gene Therapy Cold Chain Logistics market.Available Customizations:
With the given market data, the publisher offers customizations according to a company’s specific needs.This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
- Atelerix Ltd.
- Life Science Group Ltd (LSG)
- Arvato Group
- DGP Intelius Ltd.
- QuickSTAT (A Kuehne + Nagel Company)
- BIOCAIR
- TrakCel
- Cryoport UK Limited
- Patheon UK Limited (Thermo Fischer Scientific UK)
- Catalent UK
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 82 |
| Published | October 2023 |
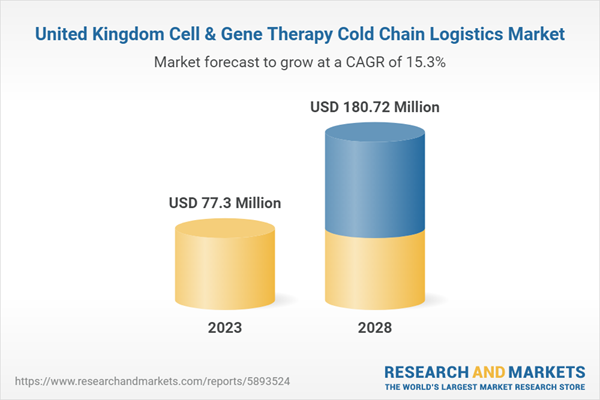
| Forecast Period | 2023 - 2028 |
| Estimated Market Value ( USD | $ 77.3 Million |
| Forecasted Market Value ( USD | $ 180.72 Million |
| Compound Annual Growth Rate | 15.2% |
| Regions Covered | United Kingdom |
| No. of Companies Mentioned | 10 |